Abstract
The Gypsum Karst of Sorbas, Almeria, southeast Spain, includes a few caves whose entrances are open and allow the entry and roosting of numerous bats. Caves are characterized by their diversity of gypsum speleothems, such as stalactites, coralloids, gypsum crusts, etc. Colored biofilms can be observed on the walls of most caves, among which the Covadura and C3 caves were studied. The objective was to determine the influence that bat mycobiomes may have on the fungal communities of biofilms. The results indicate that the fungi retrieved from white and yellow biofilms in Covadura Cave (Ascomycota, Mortierellomycota, Basidiomycota) showed a wide diversity, depending on their location, and were highly influenced by the bat population, the guano and the arthropods that thrive in the guano, while C3 Cave was more strongly influenced by soil- and arthropod-related fungi (Ascomycota, Mortierellomycota), due to the absence of roosting bats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caves are populated by a wide variety of microorganisms, including bacteria, fungi, algae, protozoa, and more, which thrive in both pristine and show caves. These microorganisms are found to be associated with biofilms that cover cave walls and sediments. Most caves present conspicuous biofilms, observable with the naked eye, in the form of round colonies, patches, or extensive microbial mats of different colors, generally white, yellow, and grey [1,2,3,4,5]. Furthermore, show caves subjected to artificial lighting exhibit wide phototrophic biofilms mainly composed of cyanobacteria and microalgae [6, 7]. The interest in the study of biofilms lies in their contribution to the physical and chemical deterioration of rocks and speleothems, which is of particular importance for the conservation of rock art that house many caves [1, 5, 7].
The case of Altamira Cave, Spain, is paradigmatic with respect to the colonization of its walls by different colored biofilms [1]. Jurado et al. [8] studied both biofilms and walls in this cave without visible microbial colonizations, detecting only bacterial DNA and no fungal DNA. The absence of fungi was further established by scanning electron microscopy [9]. This was later confirmed by González-Riancho Fernández [10]. This absence was attributed to the production of bioactive compounds with antifungal properties by bacteria that form the biofilms. In particular, many strains of Crossiella, Streptomyces, Micromonospora, Nocardia, Pseudonocardia, Lechevalieria, Kibdelosporangium, Amycolatopsis, Saccharothrix, among others, were isolated from these biofilms [11, 12]. For example, recently, two Crossiella strains isolated from Altamira white biofilms inhibited the growth of other bacteria and fungi. Their genomes showed an exclusive combination of gene clusters involved in the synthesis of lanthipeptides, lasso peptides, sactipeptides, furans, polyketide synthases, and non-ribosomal peptide synthetases [13].
Further studies corroborated the absence of fungi within cave biofilms. Martin-Pozas et al. [5] investigated yellow biofilms in Pindal Cave, Spain, applying specific CARD-FISH probes targeting Ascomycota and Basidiomycota, with negative results. Additionally, sequence analysis using fungal primers did not detect any fungal sequences, concluding that fungi were not present in the yellow biofilms. However, other authors found fungi in cave biofilms from different countries [7, 14, 15]. It should be noted that fungi are common in cave air, as well as in excrements, guano, and animal cadavers frequently found in caves and sediments [16, 17].
Many cave fungi have been related to arthropods and bats [12, 18,19,20,21], while others appear to be specific to cave ecosystems [22, 23]. Although considerable attention has been directed towards fungi in limestone and volcanic caves [2, 24,25,26], studies on gypsum caves are scarce, if not lacking.
Cunningham et al. [27] found in Lechuguilla, a gypsum cave in New Mexico, patches of fungi on deposits, and aspergilli associated with Fe-, Mn- and S-rich encrustations. Cacchio et al. [28] investigated the involvement of bacteria in the formation of speleothems in the gypsum cave Grave Grubbo, Italy. Mihajlovski et al. [29] studied the fungal diversity in gypsum efflorescences in a French cave and described the abundant presence of Isaria fumosorosea and Engyodontium album, two well-known entomopathogens [19].
Some gypsum karsts are important in Western Europe because they are excellent examples (geoheritage sites) of emerged outcrops of selenitic gypsum, which formerly corresponded to different peripheral basins around the Mediterranean with a diachronous precipitation of evaporite deposits during the Messinian age. These karstified gypsum outcrops are mainly located in the Apennines and Sicily, Italy [30], while in Spain the Gypsum Karst of Sorbas in Almeria is notable [31]. The geology, geomorphology, hydrogeology, and microclimatology of this last karst have been extensively studied in the last two decades [32,33,34,35]; however, the microbial communities of their gypsum caves are unknown.
In the gypsum karst of Sorbas, Covadura Cave harbored an abundant bat population, although this has decreased considerably in recent years, with only isolated individuals currently detected in the galleries sampled in this study. In C3 Cave there is an absence of roosting bats. Altamira and Pindal caves, located in northern Spain, do not have bats in their confined and close environments. Nonetheless, all the four caves show abundant white and yellow biofilms composed of bacteria, but no fungi were retrieved from the Altamira and Pindal caves [5, 10, 36], and unpublished data. Here, we study the fungal diversity in the biofilms that cover the walls of two gypsum caves to determine whether bat microbiomes indeed have influence on the wall biofilms.
Material and Methods
The Gypsum Karst of Sorbas
The Gypsum Karst of Sorbas, is located in the province of Almeria, southeast Spain (Fig. 1). This is formed by significant gypsiferous Messinian evaporates within a 120 m thick cyclic sequence formed by alternating gypsum and marly units, and many caves amounting to 100 km of passages, including the six most relevant caves for their extension and representativeness in the local karst hydrogeology (Covadura Cave, C3 Cave, Gypsum Cave, Water Cave, Treasure Cave and GEP Complex) [31].
Location of the Gypsum Karst of Sorbas (southern Spain) and spatial distribution of the galleries of the Covadura and C3 caves (yellow areas) in relation to the external topography. The dashed blue lines show the drainage network on the surface. The yellow areas highlighted by a red line correspond to the galleries studied in the Covadura Cave
The Covadura Cave is 120 m deep and 275 m long in the studied area, although the system has more than 4 km of galleries. The C3 Cave, at some 200 m from the Covadura Cave, is only about 3 m deep and has a length of 150 m [34]. A diversity of gypsum speleothems, such as stalactites, coralloids, gypsum crusts, etc. have been described in these caves, as well as colored biofilms (Fig. 2).
The Covadura Cave harbored a bat population of more than 5,000 bats, composed of the species Rhinolophus ferrumequinum, Rhinolophus hipposideros, and Miniopterus schreibersii. Rhinolophus euryale has occasionally been found [37]. Access to the caves is forbidden from October to March to protect the bats during the hibernation period. In Covadura Cave there has been some activation of the watercourse and partial flooding washed away guano; however, the area of wall biofilms has not been affected and may have retained previously existing fungal populations. However, through visits and sampling carried out in recent years, the disappearance of guano in the cave has been confirmed. In Cave C3, located about 200 m from Covadura Cave, there have never been bat colonies.
The climate in the Sorbas area is semi-arid Mediterranean, with a mean annual temperature and rainfall of 17 °C and 274 mm, respectively. Estimated annual potential evapotranspiration is nearly five times the mean annual precipitation, and about 80% annual rainfall during low-frequency rainstorm events, usually in the autumn. The vegetation comprises scattered shrubs and dwarf shrubs, as well as some crass perennial plants are also common. The cover of perennial vegetation is below 40 % at both study sites and is mainly made up of tussock grass. Plant interspaces are usually colonized by well-developed biocrusts dominated by lichens.
Preparation and Characterization of Samples by Scanning Electron Microscopy (SEM)
Biofilms were collected and transported at 4°C without separating them from the mineral support on which they were growing. Sample preparation for SEM analysis was carried out as described by Martin-Pozas [5]. Biofilm structures were examined under high and low vacuum conditions using an environmental scanning electron microscope Inspect (FEI, USA).
Sampling Method
In 2010 and 2022, nine white and yellow biofilm samples on the gypsum walls were collected in Covadura Cave, while only two biofilm samples from the C3 Cave were sampled in 2022 (Supplementary Figure S1). Biofilm samples were collected using sterile scalpels and preserved in Lifeguard preservation solution (Qiagen, Hilden, Germany) until their arrival in the laboratory, where they were stored at -80°C.
DNA Extraction, Sequencing and Data Processing
Genomic DNA extraction was performed using the FastDNA SPIN Kit for Soil (MP Biomedicals, Illkirch, France). DNA concentrations were quantified using a Qubit 2.0 fluorometer (Invitrogen, Carlsbad, CA, USA). High-throughput sequencing of the extracted DNA was carried out by FISABIO Institute (Valencia). The intergenic transcribed spacers (ITS) of fungal organisms were amplified according to Toju et al. [38]. The primer sequences used were ITS3_KYO2 (GATGAAGAACGYAGYRAA) and ITS4_KYO1 (TCCTCCGCTTWTTGWTWTGC), using Illumina MiSeq and 2 × 300 paired end sequencing. Sequence data have been performed using the Qiime2 pipeline [39]. Metataxonomy analysis was performed using some of the qiime2 plugins. Denoising, joining of the paired-ends, and depletion of the chimera was performed starting from data from the paired ends using DADA2 pipeline [40]. Taxonomic affiliations were assigned using the Naive Bayesian classifier integrated in the quiime2 plugins. The database used for taxonomic assignment was UNITE version 10.0 [41]. All computations and statistics were performed within the R Statistics environment [42]. Taxonomic data obtained in QIIME2 were imported into R and analyzed with the phyloseq package. From these taxonomic data, ecological roles were assigned using the FUNGuildR tool and the FUNGuild database [43]. To assess ecological alpha diversity within each sample, we used non-phylogenetic diversity indices such as Chao1, Shannon, and Simpson. These common metrics were calculated using raw species-level data with R packages phyloseq and vegan. The plots and heatmap were built using the R packages ggplot 2 and pheatmap.
Deposit of Sequences
The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database under the project number PRJNA1103123.
Results
Biofilms are mainly composed of bacteria, but fungal spores were also evident (Fig. 3). SEM images of these biofilms were similar to those obtained in the biofilms from Castañar Cave, with a high abundance of bacteria and fungi [25, 44]. The bacterial composition of the biofilms will be discussed in a further work, but we can anticipate that white and yellow biofilms in these gypsum caves are mainly composed of bacteria with a relatively high abundance of two main phyla: Actinobacteriota and Pseudomonadota, followed by Acidobacteriota and Planctomycetota.
SEM images of biofilms found on the walls of the Covadura and C3 caves. A network of bacterial structures with characteristics of Actinomycetota, the most abundant phylum, is shown in A-D images. A. White biofilm in Covadura Cave; B. White biofilm in C3 Cave; C. Yellow biofilm in Covadura Cave; D. Yellow biofilm in C3 Cave. E – F. Long smooth filaments (1.4 μm width) and spores (10-14 μm diameter) of fungi found in Covadura Cave
In the study of fungi, a total of 2,515,260 high-quality sequence reads were obtained, ranging from 8,652 to 309,474 from the white and yellow biofilms (Supplementary Table S1). In total, 422 amplicon sequence variants (ASVs) were assigned based on 99% sequence similarity. According to diversity indices, samples with the highest Chao1, Shannon, and Simpson values are found in the white and yellow biofilms from Covadura Cave, indicating greater species diversity and more uniform distribution of relative species abundance compared to samples from Cave C3 (Supplementary Table S1 and Figure S2). In summary, Covadura Cave exhibits greater species richness and diversity than C3 Cave, and specifically, the yellow biofilms exhibit higher species diversity, particularly those from 2010.
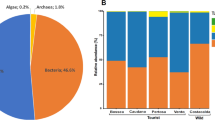
In the white biofilms collected in the Covadura Cave, Ascomycota (relative abundance 62.2% – 99.1%) and Basidiomycota (0.4% – 37.7%) were the most abundant phyla. However, yellow biofilms showed a different and variable pattern, with higher abundances of Mortierellomycota in three samples (19.2% – 97.4%), Ascomycota in four samples (39.8% – 97.0%) and Basidiomycota in the other two samples (11.0% – 20.4%). Additionally, a yellow biofilm sample presented 19.0% relative abundance of a phylum at an unclassified taxonomic rank. Negligible or no relative abundances were observed for other phyla, including unidentified sequences (Fig. 4). In general, a decrease in Ascomycota relative abundances was detected in white biofilms in 2022 (75.6 – 62.2%) compared to levels recorded in 2010 (77.3 – 99.1%), although no consistent pattern was observed in yellow biofilms, with variable relative abundances between samples. This lack of consistency was also evident in the abundances of Basidiomycota and Mortierellomycota.
In C3 Cave, the fungal community in the white biofilm was predominantly composed of Ascomycota, representing 95.9% of the relative abundance, followed by Mortierellomycota (3.8%). The yellow biofilm exhibited a reverse trend, with Mortierellomycota comprising 94.8% of the community and Ascomycota representing around 5.0%.
In all samples, the most abundant classes were Sordariomycetes, Mortierellomycetes, and Dothideomycetes. Sordariomycetes were represented in all samples from the two caves, although with variable abundance depending on the samples (1.8% – 95.4%) (Fig. 5). Mortierellomycetes were relatively abundant in the yellow biofilms of Covadura Cave (0.2% – 97.4%), and in C3 Cave (94.8%), but only appeared in a sample of white biofilms from Covadura Cave (1.6%) and in C3 (3.8%). Dothideomycetes were more abundant in the yellow biofilms collected in the Covadura Cave in 2010 (9.6% – 75.5%) than in 2022 (0% – 11.1%) as well as for the white biofilms (6.0% – 57.2% in 2010 vs 0.3% – 0.5% in 2022). An unidentified Basidiomycota taxonomic class presented relative abundances of 21.7% and 37.1% in the two white biofilms from Covadura Cave collected in 2022.
Eurotiomycetes and Agaricomycetes were relatively abundant in only one sample of Covadura Cave white biofilms collected in 2010 (16.1% and 13.0%, respectively). In the white biofilms samples collected in 2022 in the Covadura Cave, Eurotiomycetes were absent and Agaricomycetes showed low abundances (0.4 – 0.6%). In the yellow biofilms of Covadura Cave Eurotiomycetes reached low abundances in 2022 (0% – 1.3%) and in 2010 (0.3% – 2.4%) and Agaricomycetes slightly higher in 2022 (0% – 8.6%) than in 2010 (1.4% – 3.5%). In the C3 Cave, the abundances of these two classes were negligible. Relative abundances for other classes were missing or negligible, except for the 2010 white (Tremellomycetes 9.8%) and yellow biofilm samples (Leotiomycetes 5.8%, Saccharomycetes 5.7% and Malasseziomycetes 9.2%).
More than 100 fungal genera were identified, with an additional forty-six genera remaining unidentified in the Covadura and C3 caves. Significant differences were observed between white and yellow biofilms on the abundance of the genera (Fig. 6). In Covadura Cave, several genera presented relative abundances above 10% in different biofilms, but the distribution between white and yellow biofilms was different. The 2010 white biofilms were characterized by the abundant occurrence (>10%) of Engyodontium, Toxicocladosporium, Lecanicillium, Neocamarosporium and Trechispora, and in the 2022 white biofilms of Lecanicillium, Lasionectria and an unidentified Basidiomycota. The yellow biofilms showed a high abundance of Lecanicillium, Cladosporium, Podila, and Mortierella in the 2010 samples, and of Lecanicillium and Podila in the 2022 samples. Other genera also well represented (5-10%) included Alternaria, Malbranchea and Chrysosporium in white biofilms and Malassezia and Hirsutella in yellow biofilms. In the C3 Cave, only Lecanicillium and Verticillium were important (>10%) in the white biofilms and Mortierella in the yellow ones. Metacordyceps (7.2%) was identified in the white biofilms.
The most abundant and common genus in all samples was Lecanicillium, an insect pathogen, both in Covadura white and yellow biofilms, as well as in C3 white and yellow biofilms. Lecanicillium antillanum was relatively abundant (23.2%) in a yellow biofilm from 2010.
Other well-known entomopathogenic fungal genera are Engyodontium (=Beauveria), Hirsutella, Simplicillium, Metacordyceps, etc., also found in Covadura biofilms. Engyodontium was only abundant (74.1%) in a white biofilm collected in 2010, while Hirsutella (5.1%) and Simplicillium (2.6%) were found in yellow biofilms of the same year.
The Mortierellaceae include the genus Mortierella, Linnemannia, and Podila among others. M. alpina reached a relative abundance of 94.6% in the yellow biofilm of the C3 Cave and 17.0% in a 2010 yellow biofilm from the Covadura Cave. M. elongata was identified in a 2010 yellow biofilm (4.9%).
The genus Podila was established in 2020 to accommodate some Mortierella species that are difficult to identify by ITS sequences [45]. Podila was represented in yellow biofilms with 97.3% relative abundance in a yellow biofilm from 2022 and 27.5% in another from 2010.
The genus Cladosporium was relatively abundant in three 2010 yellow Covadura biofilms (5.9 –64.6%), less abundant in 2010 white biofilms (4.5 – 7.9%), and largely absent in most 2022 samples. Its abundance in the C3 Cave was also insignificant (0.0 – 0.4%), Cladosporium halotolerans was only identified in a yellow biofilm from 2010 (1.0%).
The species Verticillium leptobactrum (= Leptobacillium leptobactrum), a recognized entomopathogenic fungus, was identified in the white biofilm of C3 Cave (10.0% relative abundance) and 0.1% in a few Covadura and C3 biofilms. Metacordyceps reached 7.2% in the white biofilm of C3 Cave and 0.1% in a yellow Covadura biofilm.
Abundant genera, but only present in a unique sample of a Covadura 2010 white biofilm, were Toxicocladosporium, and Neocamarosporium. These two genera and a few others such as Catenulostroma, Libertasomyces, Xylodon, Symmetrospora, and Thelephora, were recovered in low abundances in the biofilms.
A detailed classification of fungal traits is presented in Fig. 7. There, it can be observed that the abundances of saprotrophs and pathotrophs were higher than 60% of the total relative abundances in C3, while the abundances of saprotrophs were also high in Covadura Cave, but the pathotrophs decreased to 40%. Other important traits were wood saprotroph and animal pathogen.
Discussion
Many studies reported the presence of fungi in caves, particularly in phototrophic biofilms. Predominantly, these fungi belong to the phyla Ascomycota, Zygomycota and Basidiomycota [7, 14, 46]. The dominant fungal phyla in the Covadura and C3 gypsum caves were Ascomycota, Mortierellomycota, and Basidiomycota. This is in agreement with data from other authors in karstic caves [25, 46].
The most abundant classes in the two caves were Sordariomycetes, Mortierellomycetes, and Dothideomycetes. Similar abundances of these three classes have been reported in a few caves from different countries and rock surfaces [47,48,49,50].
The Sordariomycetes are represented by the genera Lecanicillium, Engyodontium, and Lasionectria, with abundances over 10% in at least one of the samples. The genus Lecanicillium comprises insect pathogens and also parasitizes other arthropods, fungi, and plants [51]. Different species of Lecanicillium were reported in Spanish and other European caves with an abundance of arthropods [17, 18, 24, 52, 53]. However, only one species of Lecanicillium was identified, L. antillanum in the 2010 Covadura white and yellow biofilms and the C3 yellow biofilm.
The caves in the Gypsum Karst of Sorbas harbor a wide diversity of arthropods, along with bats. Indeed, many authors have recorded the occurrence of numerous arthropod taxa in these caves, including members of the orders Araneida, Anactinotrichida, Opilionida, Pseudoescorpionida, Isopoda, Quilopoda, Collembola, Psocoptera, Thysanoptera, Thysanura, Hemiptera, Coleoptera, Siphonaptera, Diptera, Diplura, Orthoptera, Trichoptera, among others [54, 55]. Most of these orders and others have been reported in bat guano in different geographical locations [56, 57]. Therefore, the abundant occurrence of Lecanicillium is probably linked to cave arthropods and bat guano.
The Mortierellomycetes, (Mortierella, Linnemannia, Podila, etc.), are saprobic organisms with a widespread distribution in a wide range of habitats [45]. Among all Mortierella species, the most commonly recovered in caves were M. alpina and M. elongata (=Linnemannia elongata) [24, 25, 59]. Many species of the genus Mortierella have been isolated from bats, dung samples collected in caves and mines, wings and bat carcasses, in addition to soils where they are abundant [16, 58,59,60]. Podila species are frequent in soils, compost and dung [45].
In addition to Mortierella, a large number of genera found in the biofilms of Covadura Cave have previously been recovered from bat guano. These include Lecanicillium, Cladosporium, Alternaria, Aureobasidium, Botrytis, Simplicillium, Debaryomyces, Talaromyces, Chrysosporium, Apiotrichum, and Cutaneotrichosporon, while only Lecanicillium, Cladosporium, Alternaria, and Aureobasidium were found in C3 Cave [21, 24, 61,62,63]. Furthermore, most of these genera and others (Filobasidium, Malassezia, Vishniacozyma, Wallemia, Comoclasthris, Cephalotrichum, Lasionectria, Solicozyma, Acremonium, etc.) were isolated from bat mycobiomes [58, 64].
The Dothideomycetes are represented by the genera Cladosporium, Toxicocladosporium, and Neocamarosporium with significant abundances. Cladosporium halotolerans, the most frequently isolated indoor species [65], was found in the two gypsum caves, and previously in other Spanish caves [17]. Other species of Cladosporium are ubiquitous plant pathogens and were also isolated from caves [17, 66]. Toxicocladosporium was detected in biofilms from stones [67], and plants [68], as well as Neocamarosporium [69, 70].
Important ecological traits observed in the genera found in the Covadura and C3 biofilms were the chitinolytic and keratinolytic activities. Chitinolytic activity is related to the occurrence of insectivorous bats and chitin remains in the guano. Chitinolytic fungal genera were identified in both caves but with a higher diversity in Covadura (Cladosporium, Alternaria, Verticillium, Aureobasidium, Acremonium, Trichoderma, Talaromyces, Botrytis, Stachybotrys, and Mortierella than in C3 (Verticillium, Aureobasidium, Acremonium, Mortierella).
Keratinolysis is common in members of the order Onygenales (Malbranchea, Chrysosporium) and Eurotiales (Cladosporium, Talaromyces). Another fungal group of interest in Covadura Cave is the class Tremellomycetes that includes the basidiomycetous yeasts Filobasidium, Apiotrichum, Vishniacozyma, Cutaneotrichosporon, and Dioszegia. These genera have been associated with bats [64, 71]. In the C3 Cave only Vishniacozyma (=Cryptococcus) was identified. Members of this genus are common in soils and plants [72, 73].
Of no less interest is the increased abundance of a few genera (Lecanicillium, Lasionectria, unidentified Basidiomycota) in the Covadura 2022 sampling of white biofilms and of Lecanicillium and Podila in the yellow biofilms. The decrease in abundance in 2022 of a few genera that were abundant in the 2010 yellow biofilms (Engyodontium, Mortierella) is noteworthy. These changes can be attributed to fluctuations in arthropod/bat populations and/or severe droughts in recent years.
When comparing the Covadura and C3 cave biofilms with those of the Altamira and Pindal limestone caves, the strongest difference was the absence of fungi in the limestone biofilms [5, 8, 10]. In the limestone caves no bat roosts were found. The entrances to the gypsum caves are open, allowing the entry and roosting of bats. Therefore, the high number of fungal genera identified in the bat microbiome by a few authors [45, 64, 74], and their finding in cave biofilms, are not surprising. Furthermore, Hathaway et al. [58] reported that bat microbiomes have an influence on the cave wall microbiome, and Kokurewicz et al. [75] stated that the number of bats was a key factor determining the occurrence of fungal spores at the hibernation site.
There is a general consensus on the influence of arthropods, bats and guano on cave fungi. Yoder et al. [76] reported the presence of entomopathogenic fungi associated to cave spiders. Vanderwolf et al. [77] studied the fungi in three caves, one populated by bats and the other two without bats. They concluded that caves with active bat hibernacula supported bat-related fungi, while caves without bats, but abundant arthropods, contained entomopathogenic fungal genera. The conclusions of this and other papers [58, 64, 74,75,76] were confirmed in this study on biofilms from gypsum caves.
In summary, the fungi recovered from the different white and yellow biofilms in Covadura Cave showed a wide diversity, with differences depending on their location and year of sampling, and were highly influenced by the presence of bats, guano, and arthropods that thrive in the guano. In the C3 Cave the occurrence of bat-related fungi was low, probably due to the absence of roosting bats, with the cave environment dominated by soil- and arthropod-related fungi.
Data Availability
The gene sequences and accompanying metadata were deposited in the Sequence Read Archive (SRA) of NCBI under the project number PRJNA1103123.
References
Saiz-Jimenez C, Cuezva S, Jurado V, Fernandez-Cortes A, Porca E, Benavente D, Cañaveras JC, Sanchez-Moral S (2011) Paleolithic art in peril: Policy and science collide at Altamira Cave. Science 334:42–43. https://doi.org/10.1126/science.1206788
Northup DE, Lavoie KH (2015) Microbial diversity and ecology of lava caves. In: Engel AS (ed) Microbial Life of Cave Systems. DeGruiter, Berlin, pp 161–192
Riquelme C, Hathaway JJM, Dapkevicius MLNE, Miller AZ, Kooser A, Northup DE, Jurado V, Fernandez O, Saiz-Jimenez C, Cheeptham N (2015) Actinobacterial diversity in volcanic caves and associated geomicrobiological interactions. Front Microbiol 6:1342. https://doi.org/10.3389/fmicb.2015.01342
Gonzalez-Pimentel JL, Miller AZ, Jurado V, Laiz L, Pereira MFC, Saiz-Jimenez C (2018) Yellow colored mats from lava tube of La Palma (Canary Islands, Spain) are dominated by metabolically active Actinobacteria. Sci Rep 8:1944. https://doi.org/10.1038/s41598-018-20393-2
Martin-Pozas T, Fernandez-Cortes A, Cuezva S, Cañaveras JC, Benavente D, Duarte E, Saiz-Jimenez C, Sanchez-Moral S (2023) New insights into the structure, microbial diversity and ecology of yellow biofilms in a Paleolithic rock art cave (Pindal Cave, Asturias, Spain). Sci Total Environ 897:165218. https://doi.org/10.1016/j.scitotenv.2023.165218
Jurado V, del Rosal Y, Gonzalez-Pimentel JL, Hermosin B, Saiz-Jimenez C (2020) Biological control of phototrophic biofilms in a show cave: The case of Nerja Cave. Appl Sci 10:3448. https://doi.org/10.3390/app10103448
Jurado V, Gonzalez-Pimentel JL, Fernandez-Cortes A, Martin-Pozas T, Ontañon R, Palacio E, Hermosin B, Sanchez-Moral S, Saiz-Jimenez C (2022) Early detection of phototrophic biofilms in the Polychrome Panel, El Castillo Cave. Spain Appl Biosci 1:40–63. https://doi.org/10.3390/applbiosci1010003
Jurado V, Fernández-Cortés A, Cuezva S, Laiz L, Cañaveras JC, Sanchez-Moral S, Saiz-Jimenez C (2009) The fungal colonization of rock art caves. Naturwissenschaften 96:1027–1034. https://doi.org/10.1007/s00114-009-0561-6
Cuezva S, Sanchez-Moral S, Saiz-Jimenez C, Cañaveras JC (2009) Microbial communities and associated mineral fabrics in Altamira Cave, Spain. Int J Speleol 38:83–92
González-Riancho Fernández, C. 2021. Análisis descriptivo y funcional de las colonias microbianas visibles que crecen en la cueva de Altamira, enfocado al diseño de medidas de control. Ph D Thesis, Universidad de Cantabria. https://repositorio.unican.es/xmlui/bitstream/handle/10902/21657/Tesis%20CGRF.pdf?sequence=1&isAllowed=y
Groth I, Vetermann R, Schuetze B, Schumann P, Saiz-Jimenez C (1999) Actinomycetes in kartic caves of Northern Spain (Altamira and Tito Bustillo). J Microbiol Methods 36:115–122. https://doi.org/10.1016/S0167-7012(99)00016-0
Sánchez-Moral, S., Cuezva, S., Fernández-Cortés, A., Janices, I., Benavente, D., Cañaveras, J.C., Élez, J., González, J.M., Jurado, V., Láiz, L., Portillo M.C., Rogerio, M.A., Sáiz-Jiménez, C. 2014. Estudio integral del estado de conservación de la cueva de Altamira y su arte paleolítico (2007 - 2009). Perspectivas futuras de conservación. Monografías. N° 24, Museo Nacional y Centro de Investigación de Altamira, 405 p.
Gonzalez-Pimentel JL, Dominguez-Moñino I, Jurado V, Laiz L, Caldeira AT, Saiz-Jimenez C (2022) The rare actinobacterium Crossiella sp. is a potential source of new bioactive compounds with activity against bacteria and fungi. Microorganisms 10:1575. https://doi.org/10.3390/microorganisms10081575
Pfendler S, Karimi B, Maron PA, Ciadamidaro L, Valot B, Bousta F, Alaoui-Sosse L, Alaoui-Sossé B, Aleya L (2018) Biofilm biodiversity in French and Swiss show caves using the metabarcoding approach: first data. Sci Total Environ 615:1207–1217. https://doi.org/10.1016/j.scitotenv.2017.10.054
Wang Y, Cheng X, Wang H, Zhou J, Liu X, Tuovinen OH (2022) The characterization of microbiome and interactions on weathered rocks in a subsurface karst cave, Central China. Front Microbiol 13:909494. https://doi.org/10.3389/fmicb.2022.909494
Nováková A, Kubátová A, Sklenár F, Hubka V (2018) Microscopic fungi on cadavers and skeletons from cave and mine environments. Czech Mycol 70:101–121
Dominguez-Moñino I, Jurado V, Rogerio-Candelera MA, Hermosin B, Saiz-Jimenez C (2021) Airborne fungi in show caves from Southern Spain. Appl Sci 11:5027. https://doi.org/10.3390/app11115027
Kubátová A, Dvořák L (2005) Entomopathogenic fungi associated with insect hibernating in underground shelters. Czech Mycol 57:221–237. https://doi.org/10.33585/cmy.57303
Jurado V, Sanchez-Moral S, Saiz-Jimenez C (2008) Entomogenous fungi and the conservation of the cultural heritage: A review. Int Biodeterior Biodegradation 62:325–330. https://doi.org/10.1016/j.ibiod.2008.05.002
Cunha AOB, Bezerra JDP, Oliveira TGL, Barbier E, Bernard E, Machado AR (2020) Living in the dark: Bat caves as hotspots of fungal diversity. PLoS One 15:e0243494. https://doi.org/10.1371/journal.pone.0243494
Vanderwolf KJ, Campbell LJ, Taylor DR, Goldberg TL, Blehert DS, Lorch JM (2021) Mycobiome traits associated with disease tolerance predict many western North American bat species will be susceptible to white-nose syndrome. Microbiol Spectr 9:e00254–e00221. https://doi.org/10.1128/Spectrum.00254-21
Martin-Sanchez PM, Nováková A, Bastian F, Alabouvette C, Saiz-Jimenez C (2012) Two new species of the genus Ochroconis, O. lascauxensis and O. anomala isolated from black stains in Lascaux Cave, France. Fungal Biol 116:574–589. https://doi.org/10.1016/j.funbio.2012.02.006
Nováková A, Hubka V, Saiz-Jimenez C, Kolarik M (2012) Aspergillus baeticus sp. nov. and Aspergillus thesauricus sp. nov.: two new species in section Usti originating from Spanish caves. Int J Syst Evol Microbiol 62:2778–2785. https://doi.org/10.1099/ijs.0.041004-0
Nováková A (2009) Microscopic fungi isolated from the Domica Cave system (Slovak Karst National Park, Slovakia). A review. Int J Speleol 38:71–82. https://doi.org/10.5038/1827-806X.38.1.8
Martin-Pozas T, Nováková A, Jurado V, Fernandez-Cortes A, Cuezva S, Saiz-Jimenez C, Sanchez-Moral S (2022) Persistence and diversity of microfungi in a high radon cave ecosystem. Front Microbiol 13:869661. https://doi.org/10.3389/fmicb.2022.869661
Biagioli F, Coleine C, Delgado-Baquerizo M, Feng Y, Saiz-Jimenez C, Selbmann L (2024) Outdoor climate drives diversity patterns of dominant microbial taxa in caves worldwide. Sci Total Environ 906:167674. https://doi.org/10.1016/j.scitotenv.2023.167674
Cunningham KI, Northup DE, Pollastro RM, Wright WG, LaRock EJ (1995) Bacteria, fungi and biokarst in Lechuguilla Cave, Carlsbad Caverns National Park, New Mexico. Environ Geol 25:2–8
Cacchio P, Ercole C, Contento R, Cappuccio G, Martinez MP, Del Gallo M, Lepidi A (2012) Involvement of bacteria in the origin of a newly described speleothem in the gypsum cave of Grave Grubbo (Crotone, Italy). J Cave Karst Stud 74:7–18. https://doi.org/10.4311/2010MB0136R
Mihajlovski A, Lepinay C, Mirval A-L, Touron S, Bousta F, Di Martino P (2019) Characterization of the archaeal and fungal diversity associated with gypsum efflorescences on the walls of the decorated Sorcerer’s prehistoric cave. Ann Microbiol 69:1071–1078. https://doi.org/10.1007/s13213-019-01506-2
De Waele J, Piccini L, Columbu A, Madonia G, Vattano M, Calligaris C, D’Angeli IM, Parise M, Chiesi M, Sivelli M, Vigna B, Zini L, Chiarini V, Sauro F, Drysdale R, Forti P (2017) Evaporite karst in Italy: a review. Int J Speleol 46:137–168. https://doi.org/10.5038/1827-806X.46.2.2107
Gázquez F, Calaforra JM (2014) The Gypsum Karst of Sorbas, Betic Chain. In: Gutiérrez F, Gutiérrez M (eds) Landscapes and Landforms of Spain. Springer, Dordrecht, pp 127–135
Calaforra JM, Pulido-Bosch A, Lopez-Chicano M (2002) Gypsum karst in the Betic Cordillera (South Spain). Carbonates Evaporites 17:134–141. https://doi.org/10.1007/BF03176479
Calaforra JM, Pulido-Bosch A (2003) Evolution of the gypsum karst of Sorbas (SE Spain). Geomorphology 50:173–180. https://doi.org/10.1016/S0169-555X(02)00213-1
Fernández Cortés A (2004) Caracterización microclimática de cavidades y análisis de la influencia antrópica de su uso turístico. Ph D Thesis. University of Almería, Spain
Sanna L, Gazquez F, Calaforra JM (2012) A geomorphological and speleological approach in the study of hydrogeology of gypsum karst of Sorbas (SE Spain). Geogr Fis Din Quat 35:153–166. https://doi.org/10.4461/GFDQ.2012.35.14
Martín Pozas, T. 2023. Papel de los microorganismos en procesos de captación y emisión de gases de efecto invernadero en ambientes subterráneos. Ph D Thesis, Universidad Complutense de Madrid. https://docta.ucm.es/bitstreams/25b96c86-7344-4da7-907e-094ad53d7e67/download
Consejería de Medio Ambiente y Ordenación del Territorio. 2016. Valores ambientales de la zona especial de conservación Karst en Yesos de Sorbas (ES6110002), Junta de Andalucía. https://www.juntadeandalucia.es/medioambiente/portal_web/web/temas_ambientales/espacios_protegidos/01_renpa/canales_figuras_proteccion/Red_Natura/2016_06_valores_ambientales_resumenes/nuevos/6110002_karst_yesos.pdf. Accessed February 1st, 2024.
Toju H, Tanabe AS, Yamamoto S, Sato H (2012) High-Coverage ITS Primers for the DNA-Based Identification of Ascomycetes and Basidiomycetes in Environmental Samples. PLoS One 7:e40863. https://doi.org/10.1371/journal.pone.0040863
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA et al (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. https://doi.org/10.1038/s41587-019-0209-9
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Abarenkov K, Zirk A, Piirmann T, Pöhönen R, Ivanov F, Nilsson RH, Kõljalg U (2021) Full UNITE+INSD dataset for eukaryotes. https://doi.org/10.15156/BIO/1281567
R Core Team. 2012. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from http://www.R-project.org/
Nguyen NH, Song Z, Bates ST, Branco S, Tedersoo L, Menke J, Schilling JS, Kennedy PG (2016) FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248. https://doi.org/10.1016/j.funeco.2015.06.006
Martin-Pozas T, Cuezva S, Fernandez-Cortes A, Benavente D, Saiz-Jimenez C, Sanchez-Moral S (2023) Prokaryotic communities inhabiting a high-radon subterranean ecosystem (Castañar Cave, Spain): Environmental and substrate-driven controls. Microbiol Res 277:127511. https://doi.org/10.1016/j.micres.2023.127511
Telagathoti A, Probst M, Mandolini E, Peintner U (2022) Mortierellaceae from subalpine and alpine habitats: new species of Entomortierella, Linnemannia, Mortierella, Podila and Tyroliella gen. nov. Stud Mycol 103:25–58. https://doi.org/10.3114/sim.2022.103.02
Biagioli F, Coleine C, Piano E, Nicolosi G, Poli A, Prigione V, Zanellati A, Varese C, Isaia M, Selbmann L (2023) Microbial diversity and proxy species for human impact in Italian karst caves. Sci Rep 13:689. https://doi.org/10.1038/s41598-022-26511-5
Man B, Wang H, Yun Y, Xiang X, Wang R, Duan Y, Cheng X (2018) Diversity of fungal communities in Heshang Cave of Central China revealed by mycobiome-sequencing. Front Microbiol 9:1400. https://doi.org/10.3389/fmicb.2018.01400
Zhang Z-F, Cai L (2019) Substrate and spatial variables are major determinants of fungal community in karst caves in Southwest China. J Biogeogr 46:1504–1518. https://doi.org/10.1111/jbi.13594
Bontemps A, Prigent-Combaret C, Guillmot A, Hugoni M, Moënne-Loccoz Y (2023) Dark-zone alterations expand throughout Paleolithic Lascaux Cave despite spatial heterogeneity of the cave microbiome. Environ Microbiome 18:31. https://doi.org/10.1186/s40793-023-00488-8
Ruibal C, Platas G, Bills GF (2008) High diversity and morphological convergence among melanised fungi from rock formations in the Central Mountain System of Spain. Persoonia 21:93–110. https://doi.org/10.3767/003158508X371379
Zare R, Gams W (2001) A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium. Nova Hedwigia 73:1–50. https://doi.org/10.1127/nova.hedwigia/71/2001/1
Bastian F, Alabouvette C, Saiz-Jimenez C (2009) The impact of arthropods on fungal community structure in Lascaux Cave. J Appl Microbiol 106:1456–1462. https://doi.org/10.1111/j.1365-2672.2008.04121.x
Sanchez-Moral S, Jurado V, Fernandez-Cortes A, Cuezva S, Martin-Pozas T, Gonzalez-Pimentel JL, Ontañon R, Saiz-Jimenez C (2021) Environment-driven control of fungi in subterranean ecosystems: the case of La Garma cave (northern Spain). Int Microbiol 24:573–591. https://doi.org/10.1007/s10123-021-00193-x
Ruiz-Portero C, Barranco P, Fernández-Cortés A, Tinaut A, Calaforra JM (2002) Aproximación al conocimiento de la entomofauna de la Cueva del Yeso (Sorbas, Almería). Bol SEDECK 3:16–25
Barranco P, Tinaut A, Baena M (2008) Entomofauna cavernícola de Andalucía. In: Calaforra Chordi JM, Berrocal Pérez JA (eds) El Karst de Andalucía. Consejería de Medio Ambiente de la Junta de Andalucia, Sevilla
Palacios-Vargas JG, Castaño-Meneses G, Estrada DA (2011) Diversity and dynamics of microarthropods from different biotopes of Las Sardinas cave (Mexico). Subterr Biol 9:113–126. https://doi.org/10.3897/subtbiol.9.2514
Pellegrini TG, Ferreiras RL (2013) Structure and interactions in a cave guano-soil continuum community. Eur J Soil Biol 57:19–26. https://doi.org/10.1016/j.ejsobi.2013.03.003
Hathaway JJM, Salazar-Hamm PS, Caimi NA, Natvig DO, Buecher DC, Northup DA (2024) Comparison of fungal and bacterial microbiomes of bats and their cave roosting environments at El Malpais National Monument, New Mexico, USA. Geomicrobiol J 41:82–97. https://doi.org/10.1080/01490451.2023.2283427
Jurado V, Porca E, Cuezva S, Fernandez-Cortes A, Sanchez-Moral S, Saiz-Jimenez C (2010) Fungal outbreak in a show cave. Sci Total Environ 408:36323638. https://doi.org/10.1016/j.scitotenv.2010.04.057
Ozimek E, Hanaka A (2021) Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 11:7. https://doi.org/10.3390/agriculture11010007
Nováková A, Kolarik M (2010) Chrysosporium speluncarum, a new species resembling Ajellomyces capsulatus, obtained from bat guano in caves of temperate Europe. Mycol Prog 9:253–260. https://doi.org/10.1007/s11557-009-0634-0
Takashima M, Kurakado S, Cho O, Kikuchi K, Sugiyama J, Sugita T (2020) Description of four Apiotrichum and two Cutaneotrichosporon species isolated from guano samples from bat-inhabited caves in Japan. Int J Syst Evol Microbiol 70:4458–4469. https://doi.org/10.1099/ijsem.0.004277
Dimkic I, Fira D, Janakiev T, Kabic J, Stupar M, Nenadic M, Unkovic N, Grbic ML (2021) The microbiome of bat guano: for what is this knowledge important? Appl Microbiol Biotechnol 105:1407–1419. https://doi.org/10.1007/s00253-021-11143-y
Becker P, van den Eynde C, Baert F, D’hooge E, De Pauw R, Normand A-C, Piarroux R, Stubbe D (2023) Remarkable fungal biodiversity on northern Belgium bats and hibernacula. Mycologia 115:484–498. https://doi.org/10.1080/00275514.2023.2213138
Bensch K, Groenewald JZ, Meijer M, Dijksterhuis J, Jurjevic Z, Andersen B, Houbraken J, Crous JW, Samson RA (2018) Cladosporium species in indoor environments. Stud Mycol 89:177–301. https://doi.org/10.1016/j.simyco.2018.03.002
Pereira MLS, Carvalho JLVR, Lima JMS, Barbier E, Bernard E, Bezarra JDP, Souza-Motta CM (2022) Richness of Cladosporium in a tropical bat cave with the description of two new species. Mycol Prog 21:345–357. https://doi.org/10.1007/s11557-021-01760-2
Wang Y, Zhang H, Liu X, Song W (2021) Fungal communities in the biofilms colonizing the basalt sculptures of the Leizhou Stone Dogs and assessment of a conservation measure. Herit Sci 9:36. https://doi.org/10.1186/s40494-021-00508-1
Cruywagen EM, Crous PW, Roux J, Slippers B, Wingfield MJ (2015) Fungi associated with black mould on baobab trees in southern Africa. Anton Leeuw 108:85–95. https://doi.org/10.1007/s10482-015-0466-7
Li J-L, Sun X, Zheng Y, Lü P-P, Wang Y-L, Guo L-D (2020) Diversity and community of culturable endophytic fungi from stems and roots of desert halophytes in northwest China. MycoKeys 62:75–95. https://doi.org/10.3897/mycokeys.62.38923
Moghaddam MS, Safaie N, Soltani J, Hagh-Doust N (2021) Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant Physiol Biochem 160:225–238. https://doi.org/10.1016/j.plaphy.2021.01.022
Borzecka J, Piecuch A, Kokurewicz T, Lavoie KH, Ogórek R (2021) Greater mouse-eared bats (Myotis myotis) hibernating in the Nietoperek bat reserve (Poland) as a vector of airborne culturable fungi. Biology 10:593. https://doi.org/10.3390/biology10070593
Yurkov AM (2018) Yeasts of the soil – obscure but precious. Yeast 35:369–378. https://doi.org/10.1002/yea.3310
Li A-H, Yuan F-X, Groenewald M, Bensch K, Yurkov AM, Li K, Han P-J, Guo L-D, Aime MC, Sampaio JP, Jindamorakot S, Turchetti B, Inacio J, Fungsin B, Wang Q-M, Bai F-Y (2020) Diversity and phylogeny of basidiomycetous yeasts from plant leaves and soil: Proposal of two new orders, three new families, eight new genera and one hundred and seven new species. Stud Mycol 96:17–140. https://doi.org/10.1016/j.simyco.2020.01.002
Kearns PJ, Winter AS, Woodhams DC, Northup DE (2023) The mycobiome of bats in the American southwest is structured by geography, bat species, and behavior. Microb Ecol 86:1565–1574. https://doi.org/10.1007/s00248-023-02230-w
Kokurewicz T, Ogórek R, Pusz W, Matkowski K (2016) Bats increase the number of cultivable airborne fungi in the “Nietoperek” bat reserve in Western Poland. Microb Ecol 72:36–48. https://doi.org/10.1007/s00248-016-0763-3
Yoder JA, Benoit JB, Christensen BS, Croxall TJ, Hobbs III HH (2009) Entomopathogenic fungi carried by the cave orb weaver spider, Meta ovalis (Araneae, Tetragnathidae), with implications for mycoflora transer to cave crickets. J Cave Karst Stud 71:116–120
Vanderwolf KJ, Malloch D, Ivanova NV, McAlpine DF (2016) Lack of cave-associated mammals influences the fungal assemblages of insular solution caves in eastern Canada. J Cave Karst Stud 78:198–207. https://doi.org/10.4311/2016MB0122
Acknowledgments
This is a contribution from CSIC Interdisciplinary Thematic Platform Open Heritage: Research and Society (PTI-PAIS). The authors wish to thank reviewers for their comments and suggestions.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research was supported by the Spanish Ministry of Science and Innovation through projects PID2019-110603RB-I00 and PID2020-114978GB-I00, MCIN/AEI/FEDER, UE/10.13039/501100011033.
Author information
Authors and Affiliations
Contributions
Conceptualization: VJ and CSJ; investigation: VJ, TMP, SSM, AFC, and JMC; writing—original draft preparation: CSJ, VJ, and TMP; writing—review and editing: CSJ, VJ, and TMP. All authors contributed to the interpretation of the data and provided significant input to the final manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Supplementary Material 1:
(PDF 433 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jurado, V., Martin-Pozas, T., Fernandez-Cortes, A. et al. Gypsum Cave Biofilm Communities are Strongly Influenced by Bat- And Arthropod-Related Fungi. Microb Ecol 87, 80 (2024). https://doi.org/10.1007/s00248-024-02395-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00248-024-02395-y