Abstract
Epiphytic bacteria constitute a vital component of aquatic ecosystems, pivotal in regulating elemental cycling. Despite their significance, the diversity and functions of epiphytic bacterial communities adhering to various submerged macrophytes remain largely unexplored. In this study, we employed a metagenomic approach to investigate the diversity and function of epiphytic bacterial communities associated with six submerged macrophytes: Ceratophyllum demersum, Hydrilla verticillata, Myriophyllum verticillatum, Potamogeton lucens, Stuckenia pectinata, and Najas marina. The results revealed that the predominant epiphytic bacterial species for each plant type included Pseudomonas spp., Microbacterium spp., and Stenotrophomonas rhizophila. Multiple comparisons and linear discriminant analysis effect size indicated a significant divergence in the community composition of epiphytic bacteria among the six submerged macrophytes, with 0.3–1% of species uniquely identified. Epiphytic bacterial richness associated with S. pectinata significantly differed from that of both C. demersum and H. verticillata, although no significant differences were observed in diversity and evenness. Functionally, notable variations were observed in the relative abundances of genes associated with carbon, nitrogen, and phosphorus cycling within epiphytic bacterial communities on the submerged macrophyte hosts. Among these communities, H. verticillata exhibited enrichment in genes related to the 3-hydroxypropionate bicycle and nitrogen assimilation, translocation, and denitrification. Conversely, M. verticillatum showcased enrichment in genes linked to the reductive citric acid cycle (Arnon-Buchanan cycle), reductive pentose phosphate cycle (Calvin cycle), polyphosphate degradation, and organic nitrogen metabolism. In summary, our findings offer valuable insights into the diversity and function of epiphytic bacteria on submerged macrophyte leaves, shedding light on their roles in lake ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Submerged macrophytes, as major primary producers in lake ecosystems, play a crucial role in shaping the structure and functioning of shallow lake ecosystems. The leaves of these submerged macrophytes offer diverse ecological niches for microorganisms [1]. Epiphytic bacterial communities associated with these macrophytes influence plant physiology and nutrient cycling in lake ecosystems, encompassing processes such as carbon, nitrogen, and phosphorus uptake and transformation, as well as heavy metal adsorption [2,3,4]. Thus, understanding the composition and function of epiphytic bacterial communities on submerged plant leaves is crucial for comprehending nutrient cycling in lakes.
The structure of epiphytic bacterial communities adhering to seaweed and terrestrial plants typically displays host-specific characteristics demonstrating a strong filtering effect of host plants on epiphytic bacteria [5, 6], revealing that foliar-attached bacterial communities in arid deserts primarily consist of Actinobacteria, Firmicutes, and Proteobacteria. Liu et al. reported significant diversity differences in leaf surface epiphytic bacterial communities among nine woodland tree species, displaying varying functional traits related to plant species [6]. Proteobacteria, Bacteroidota, Verrucomicrobia, Planctomycetota, Firmicutes, Patescibacteria, and Cyanobacteria constitute the core of epiphytic bacterial communities attached to marine macroalgae [7]. However, epiphytic bacterial communities on different algae surfaces exhibit host specificity [5, 8]. Despite extensive studies on terrestrial plants and marine algae [6, 9,10,11,12], the exploration of aquatic epiphyton gained momentum only in the early twentieth century. Consequently, there is a substantial knowledge gap regarding the composition and variability of epiphytic bacterial communities associated with different submerged macrophytes in freshwater lakes. Goldsborough et al. proposed that leaf surfaces of submerged macrophytes are not neutral substrates for epiphytic bacteria [13]. The establishment of a plant-bacterial interface depends on the physicochemical properties of plant leaves and the ability of bacteria to adapt to and modify this environment. Thus, morphological or metabolic differences in plant leaf surfaces can result in host-specific epiphytic bacterial communities [14]. For example, it was suggested that plant foliar sugar content limits the epiphytic bacterial population size under favorable environmental conditions [15]. Hempel et al. examined bacterial community composition on leaves of Chara aspera and Myriophyllum spicatum using FISH, revealing variability in colonizing bacterial communities [16]. He et al. found significant differences in epiphytic bacterial communities in the leaves of the high-phenolic plant Hydrilla verticillata and the low-phenolic plant Vallisneria natans using terminal restriction fragment length polymorphism (T-RFLP) and clone library analysis targeting bacterial 16S rRNA genes [17]. Differences in phenolic content among plant species may be a major factor contributing to the differences in epiphytic bacterial communities [3]. In contrast, epiphytic bacterial communities of M. spicatum from different locations exhibited similar core bacterial species [18], suggesting that conspecifics maintain similar core epiphytic bacterial communities despite environmental variations. However, our knowledge on this topic remains limited.
Epiphytic bacteria play a crucial role in the metabolic cycle of freshwater ecosystems, demonstrating diverse mechanisms of nitrogen metabolism, including nitrogen fixation, nitrification, and denitrification in bacterial communities on plant stems and leaves [19]. Yan et al. confirmed the excellent denitrification capacity of three submerged epiphytic bacterial communities [20], and Sun et al. identified functions related to nitrogen metabolism in M. spicatum epiphytic bacteria. While these studies deepen our understanding of the biogeochemical cycle driven by epiphytic bacteria on submerged macrophytes in lake ecosystems, most previous research has focused on comparisons between only two or three submerged macrophytes [18]. For example, He et al. analyzed differences in the epiphytic bacterial communities of two submerged macrophytes, H. verticillata and V. natans [17]. With the advancement of macrogenome sequencing technology, high-throughput sequencing allows for a more comprehensive exploration of epiphytic bacterial community diversity on submerged macrophytes and the determination of metabolic pathways of environmental microorganisms [21]. Nevertheless, the functions of epiphytic bacterial communities on submerged macrophytes remain largely unknown.
This study aims to explore the compositional and functional variability of epiphytic bacterial communities on the leaf surfaces of six different species of submerged macrophytes using macrogenomics. Our objectives include understanding the (1) structure and diversity of community composition of epiphytic bacteria in different plants and their correlation with plant complexity, (2) differences in the structure of host plant-attached microbial communities, and (3) differences in metabolic genes associated with nutrient cycling in epiphytic bacterial communities among different host plants. Investigating the structure and function of epiphytic bacterial communities on submerged plant communities not only enriches the understanding of their biodiversity but also provides insights into the role of submerged macrophytes in lake ecological restoration.
Materials and Methods
Study Area and Sampling
Weining Caohai (26° 47′ 35″ N, 104° 9′ 23″ E) in Guizhou Province is a complete and representative karst plateau wetland ecosystem, formed by water accumulation in karst basins. It stands as one of the few natural freshwater lakes at the same latitude on the plateau. Located in the hinterland of the Wumeng Mountains on the Yunnan-Guizhou Plateau, on the northwest edge of Guizhou Province, it spans 25 km2 with an elevation of 2171.7 m. Known as the “underwater forest”, Caohai Lake is abundant in submerged vegetation. In October 2021, we gathered 36 biofilm samples from the leaves of six submerged plant species at a 0.5 m water depth from six sites in the southwestern part of Weining Caohai, where aquatic plants flourish. Each site harbored six species of submerged plants, including Ceratophyllum demersum, Hydrilla verticillata, Myriophyllum verticillatum, Potamogeton lucens, Stuckenia pectinata, and Najas marina. Sampling sites, spaced 1 km apart with similar hydrogeological conditions, hosted 3–5 replicate plants of each species. Approximately 10 g of leaves were cut and transferred to 500 ml sterile polyethylene bottles containing 400 ml of 0.05 M phosphate-buffered saline (PBS, pH = 7.4) for epiphytic bacteria analysis [22]. All leaf samples were collected in triplicate, mixed, stored on ice packs, and promptly returned to the laboratory. The samples were sonicated for 3 min, shaken for 30 min, and then sonicated again for 3 min before being retrieved with forceps. An aliquot of the remaining eluate (100 ml) was then filtered through a 0.22 μm membrane to obtain epiphytic bacteria samples.
DNA Extraction and PCR Amplification and Sequencing
DNA extraction from the collected leaf epiphytic bacteria samples utilized the E.Z.N.A. ® Soil DNA Kit (Omega Biotek, Norcross, GA, USA). DNA concentration was measured using a Quantus Fluorometer (Picogreen), DNA purity using a NanoDrop2000, and DNA integrity using 1% agarose gel electrophoresis. Extracted DNA was fragmented using a Covaris M220 Focused-ultrasonicator (Genomics, China) and screened for fragments of approximately 400 bp. Paired-end libraries were constructed using NEXTFLEX Rapid DNA-Seq (Bio Scientific, Austin, TX, USA) and sequenced using NovaSeq Reagent Kits at Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). Macrogenome sequencing was performed using an Illumina NovaSeq 6000 (Illumina, USA) platform.
Sequence Data Processing
Raw data underwent quality control using Fastp software; reads were aligned to host plant DNA sequences with Burrows-Wheeler Alignment (BWA) software, and highly similar contaminated reads were eliminated. Optimized sequences were spliced and assembled with MEGAHIT based on succinct de Bruijn graphs, filtering contigs ≥ 400 bp as the final assembly results. Open reading frame (ORF) prediction for assembled contigs utilized Prodigal v2.6.3. Predicted gene sequences were clustered with CD-HIT software to construct a non-redundant gene set. SOAPaligner software compared high-quality reads of each sample with the non-redundant gene set (95% identity), and gene abundance was counted. Species annotation results were obtained from the corresponding taxonomic information database of NR (non-redundant protein sequence database). Gene abundance at each taxonomic level, i.e., domain, kingdom, phylum, class, order, family, genus, and species, was calculated. A P < 0.05 was considered statistically significant.
Statistical Analyses
Differences in gene abundance and α-diversity of epiphytic bacteria between groups were analyzed using the Kruskal-Wallis rank-sum tests, followed by the Tukey-Kramer post hoc tests. Dominant species and functional differences in epiphytic bacteria of different plants were determined using the Kruskal-Wallis test (nonparametric approach) [23]. Venn diagrams were generated to analyze the shared and endemic epiphytic bacteria among the six submerged macrophytes. Principal coordinate analysis (PCoA) and non-metric multidimensional scaling analysis (NMDS) based on the Bray-Curtis distance were performed using the “vegan” package. Beta diversity matrix calculations were performed to evaluate differences in the detection of epiphytic bacteria at the species level [24]. Linear discriminant analysis effect size (LEfSe) identified epiphytic bacterial species differing between groups, with LDA > 3.5 as the threshold value [25]. To explore bacterial community metabolism, BLASTP was used with the Kyoto Encyclopedia of Genes and Genomes (KEGG) database to obtain KEGG annotation profiles corresponding to genes and potential metabolic pathways. The total number of identified protein features (i.e., predicted protein-coding regions) was used to normalize the relative abundance of metabolism-related genes and to generate heatmap plots to compare the abundance of genes in different epiphytic bacteria. All analyses were performed using R 4.1.2.
Results
Community Structure and Diversity of Epiphytic Bacteria
Metagenomic data comprised 1,819,941,160 raw reads, with 1,767,793,646 clean reads generated through the trimming and filtering of lower-quality data. After quality control, the sequence length of clean reads accounted for approximately 97% of the original sequence length, indicating high overall data quality. MEGAHIT splicing yielded 127,876,660 assembled sequences (contigs), with an average total sequence length of 261 Mb per sample. The coverage was close to 1, and the Rarefaction curve of the species exhibited a plateau, indicating sufficient sample size and data depth. Epiphytic bacterial richness (Chao1) attached to S. pectinata significantly surpassed that of C. demersum and H. verticillata (P < 0.05). The Shannon and Shannon even indices showed that the diversity and evenness of the S. pectinata epiphytic bacterial community were more diverse and homogeneous than those of C. demersum, though not significantly different (P > 0.05) (Fig. 1).
Alpha diversity of plant epiphytic bacterial samples estimated by a Chao index, b Shannon index, and c Shannon even index. Each box indicates the 3rd and 1st quartiles of the top and bottom boundary value ranges, respectively, and the black line within each box indicates the median value. Statistical comparison of indicated data was performed using the Kruskal-Wallis rank-sum test with *(P < 0.05). N, N. marina; P, P. lucens; M, M. verticillatum; C, C. demersum; H, H. verticillata; S, S. pectinata; there were 6 replicates for each plant
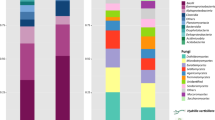
Based on comparisons with the NR database, 149 bacterial phyla were detected, encompassing 240 classes, 413 orders, 814 families, 3312 genera, and 27,336 species. In 36 samples, Proteobacteria was the prevailing phylum across all plants. Pseudomonadaceae was associated with each plant, at varying percentages: C. demersum (47.05%), H. verticillata (26.45%), and N. marina (15.45%) (Fig. 2a). The dominant family differed for M. verticillatum, P. lucens, and S. pectinata, being Microbacteriaceae (16.75%), Micrococcaceae (11.45%), and Xanthomonadaceae (10.23%), respectively. While the composition of epiphytic bacterial communities was similar, the abundance varied among plants at the genus level. For example, the abundance of Pseudomonas was the highest in C. demersum, H. verticillata, and N. marina (56.26%, 29.10%, and 20.26%, respectively), while Microbacterium was the most abundant in M. verticillatum and P. lucens (17.01% and 11.71%, respectively), and Stenotrophomonas dominated in S. pectinata (12.79%). At the species taxonomic level, C. demersum and H. verticillata were dominated by Pseudomonas spp. (26.63% and 14.67%, respectively); M. verticillatum by species of Pseudomonas spp. (7.27%), Microbacterium (5.47%), and Sphingomonas (4.45%); P. lucens by Microbacterium (2.25%); S. pectinata by Stenotrophomonas_rhizophila (6.97%); and N. marina by Pseudomonas spp. (8.90%) and Massilia sp. (5.66%).
The habitat heterogeneity hypothesis holds that habitat complexity may positively affect the abundance of attached organisms, while the microhabitat hypothesis holds that plant complexity has an important impact on the abundance of attached organisms, community diversity, and species composition changes [26, 27]. Fractal dimension was introduced to characterize plant complexity [28, 29]. Correlation analysis was used to explore the correlation between the diversity of epiphytic bacterial communities and the leaf shape dimension of six submerged plants (Fig. S1). The fractal dimension was only significantly correlated with the Shannon index (P < 0.05).
Differences in Epiphytic Bacterial Communities of Different Submerged Macrophytes
A Venn diagram analyzing epiphytic bacterial communities at the family to genus taxonomic levels revealed one unique family present in C. demersum (Candidatus_Actinomarinaceae), P. lucens (Candidatus_Niyogibacteria), and S. pectinata (Defluviitaleaceae) (Fig. S2a and b). At the genus level, P. lucens displayed the highest number (22) of unique bacteria, with Mesoplasma accounting for the largest proportion (14.67%). At the species level, the number of species endemic to each plant was as follows: C. demersum (249), H. verticillata (136), M. verticillatum (94), P. lucens (303), S. pectinata (271), and N. marina (162) (Fig. 2b). PCoA and analysis of similarities (ANOSIM) indicated statistically significant differences in the composition of epiphytic bacterial communities among plant species (R = 0.4212, P = 0.001) (Fig. 3a). This result was confirmed using NMDS (stress = 0.150) (Fig. 3b).
a Principal component analysis (PCoA) and b non-metric multidimensional scaling (NMDS) analysis showing differences in the composition of the epiphytic bacterial communities of the six submerged macrophytes at the species level (for Bray-Curtis). N, N. marina; P, P. lucens; M, M. verticillatum; C, C. demersum; H, H. verticillata; S, S. pectinata
LEfSe revealed detailed differences in the distribution of epiphytic bacterial taxa from the phylum to genus levels based on an LDA score > 3.5 (P < 0.05) (Fig. 4). Key bacterial species with significant abundance differences in the six plants included: C. demersum (Proteobacteria, Gammaproteobacteria, Pantoea, Pseudomonas, and Pneumatobacteria), H. verticillata (Comamonadaceae, Acidophilus, Rhizobacteria, and Soilobacterium spp.), M. verticillatum (Proteobacteria), P. lucens (Microbacterium, Branchiobacterium, Cyanobacteria phylum Roseofilum, Polycoccus, Proteobacteria, Sphingomonas spp.), S. pectinata (Flavobacterium spp., Chloroflexi, and Verrucomicrobia), and N. marina (Pseudomonas and Massilia_sp.). Multiple group comparisons revealed species with significant abundance differences among the six submerged macrophytes (Fig. S3).
Different colored nodes in the LEfSe plot indicate microbial taxa that are significantly enriched in the corresponding group and have a significant effect on intergroup differences; light yellow nodes indicate microbial taxa that are not significantly different in any of the different subgroups or have no significant effect on intergroup differences. N, N. marina; P, P. lucens; M, M. verticillatum; C, C. demersum; H, H. verticillata; S, S. pectinata
Within each group, there were unique bacterial taxa at the species level (Fig. S4). Species present at < 1% were combined as “other.” For each plant species, the bacterial species with the richest proportion was as follows: C. demersum (Spirochaetales_bacterium_NM-380-WT-3C1 [9.88%]), H. verticillata (Variovorax_sp._HH01 [55.04%]), M. verticillatum (Actinomyces_sp._S4-C9 [6.53%]), P. lucens (Acinetobacter_sp._ANC_4641 [13.52%]), S. pectinata (Lysinibacillus_telephonicus [4.18%]), and N. marina (Rhizobium_sp._Leaf68 [13.27%]).
Analysis of the Main Metabolic Functions of the Epiphytic Bacteria
To elucidate the potential role of epiphytic bacteria in energy metabolism, we compared macro-genomic data with the KEGG database to obtain gene abundances associated with the carbon, nitrogen, and phosphorus cycles. The outcomes are then classified into different pathways.
Five carbon fixation pathways were identified, with the reductive citric acid cycle (Arnon-Buchanan cycle), represented by ACO, acnB, E4.2.1.2A/B, ppc, PC, ppdK, pps, sdhA/B/C, and sucC/D, being the most widely distributed. This was followed by the reductive pentose phosphate cycle (Calvin cycle) (IDH1, GAPDH, mdh, rpiA, rpiB, and E2.2.1.1), reductive acetyl-CoA pathway (Wood-Ljungdahl pathway) (fhs, FBP, FBA, fold, and metF), 3-hydroxypropionate bicycle (MUT and accA/C), and hydroxypropionate-hydroxybutylate cycle (ACAT) (Fig. 5). Overall, the abundance of carbon cycle–encoding genes in the epiphytic bacterial community of M. verticillatum exceeded that in the epiphytic bacterial communities of other plant species, especially the gene encoding methylmalonyl-CoA mutase [30]. The gene encoding pyruvate, orthophosphate dikinase (ppdK) was significantly enriched in the epiphytic bacterial community of S. pectinata, while that encoding aconitate hydratase 2/2-methylisocitrate dehydratase (acnB) was enriched in C. demersum. Genes involved in these pathways were primarily contributed by Pseudomonadaceae, Micrococcaceae, Microbacteriaceae, and Sphingomonadaceae (Fig. S5a).
Top 50 functional genes involved in C, N, and P abundance in six plant epiphytic bacteria, with analysis of variance of count data (given using log2 normalized counts) used to compare gene abundance across plants. The depth of color in the heat map represents the abundance of genes. N, N. marina; P, P. lucens; M, M. verticillatum; C, C. demersum; H, H. verticillata; S, S. pectinata
Four nitrogen metabolic pathways were identified: organic nitrogen metabolism, nitrate assimilation/nitrogen transport, assimilatory nitrate reduction (ANRA), and dissimilatory nitrate reduction (DNRA) (Fig. 5). Enrichment in the gene encoding glutamate synthase (gltB) in the epiphytic bacteria of S. pectinata suggests a crucial role for the organic nitrogen metabolism pathway in this plant. Nitrogen metabolism in C. demersum was influenced by the positive enrichment of genes encoding glutamine synthetase (glnA) and glutamate dehydrogenase (NADP+) (gdhA) (P < 0.05). Enrichment of the gene encoding glutamate synthase (NADPH) (gltD) in M. verticillatum indicates an active role of organic nitrogen metabolism in this plant. Pseudomonadaceae, Microbacteriaceae, and Micrococcaceae were found to be responsible for the organic nitrogen metabolism pathway, while Pseudomonadaceae, Oxalobacteraceae, and Bradyrhizobiaceae were associated with the nitrate assimilation/nitrogen transport (Fig. S5b). Genes encoded by Microbacteriaceae and Micrococcaceae were linked to the ANRA pathway, and genes encoded by Micrococcaceae were associated with the DNRA pathway.
Five phosphorus metabolic pathways were identified: polyphosphate degradation, inorganic P solubilization, organic P mineralization, polyphosphate synthesis, and transporters (Fig. 5). The significant enrichment in the gene encoding quinoprotein glucose dehydrogenase (gcd) in C. demersum suggests the occurrence of polyphosphate ATP pathway degradation in this plant. Genes encoding NAD+ kinase (ppnK) and polyphosphate kinase (ppk2), responsible for polyphosphate degradation and organic P mineralization, were enriched in M. verticillatum, indicating phosphorylation in this plant. The gene encoding alkaline phosphatase (phoA) was enriched in S. pectinate and that of polyphosphate synthesis (ppk) in P. lucens. The gene encoding glycerophosphodiester phosphodiesterase (glpQ), responsible for transporters, was enriched in M. verticillatum. Pseudomonadaceae and Micrococcaceae were attached to transporters and polyphosphate synthesis pathways, whereas alkaline phosphatases (phoA/D) in the organic P mineralization pathway were associated with Oxalobacteraceae, Moraxellaceae, and Xanthomonadaceae. Genes attached to the polyphosphate degradation pathway (ppnk/ppk2) were mainly contributed by Pseudomonadaceae, Sphingomonadaceae, and Xanthomonadaceae, while genes involved in the inorganic P solubilization pathway were mainly contributed by Pseudomonadaceae, Xanthomonadaceae, Moraxellaceae, and Micrococcaceae. The presence of genes encoded by bacteria showed extensive phosphorus-scavenging mechanisms of these heterotrophic bacteria (Fig. S5c).
Discussion
In this study, Proteobacteria emerged as the most dominant phylum within the epiphytic bacterial communities of the six submerged macrophytes. Particularly noteworthy was the significantly higher abundance of Proteobacteria associated with C. demersum compared with that in the other five plant species. The α-diversity of the epiphytic bacterial communities exhibited significant differences among certain plant species, with Proteobacteria prevailing as the dominant taxon at the phylum level. Proteobacteria, known for its wide environmental distribution, plays a crucial role in facilitating bacterial colonization and biofilm formation [12]. It is a common phylum in the plant epiphytic bacteria community [17, 31, 32], as reported in previous studies. Notably, the genus Pseudomonas dominated the epiphytic bacterial communities in various plants, such as C. demersum, M. verticillatum, P. lucens, and S. pectinata, all exhibiting a robust capability for phosphorus solubilization in eutrophic water bodies [6, 33, 34].
Plant species are an important factor contributing to differences in the community structure of epiphytic bacteria [35]. As an important vegetative organ of submerged macroplants, leaves have a variety of functional characteristics, and the size, shape, and age of leaves also affect the structure and number of attached organisms [36]. Plant complexity has a positive effect on the biomass of surrounding plants [37, 38]. For epiphytic bacterial communities, Levi et al. compared two types of epiphytic plants and their epiphytic bacterial communities, Callitriche genus with complex morphology had higher abundance and evenness than Sparganium emersum epiphytic bacterial community with simple morphology [14]. Grossart et al. found that highly complex plants would reduce the shear stress of water, thus affecting the richness and diversity of microbial communities [39]. In our study, however, plant morphological complexity was only significantly associated with the diversity of the epiphytic bacterial community.
Furthermore, large freshwater plants can provide nutrients through secretions or the diffusion of photosynthetic products such as polyphenols and cyclic sulfur compounds to produce allelopathy supporting diverse bacterial communities [40, 41] and increasing the diversity of bacterial communities. For example, the secretions of Hydrilla verticillata promote positive interactions between bacteria and fungi, increasing the α-diversity of epiphytic bacterial communities [42]. By comparing the biomass differences between natural and artificial substrate communities, authors considered that nutrients and allelopathic substances released by natural plants are the main reasons for the significant differences [37, 43]. Exudate metabolites and secondary metabolites on plant surfaces are not the same [44]. Zhu et al. compared the allelopathic substances of five submerged plants, such as Sophora and Ceratina, which act as inhibitors or promoters of bacterial growth and lead to the colonization selection of specific epiphytic bacteria [45]. Phenolic compounds in allelopathic substances represent many secondary plant metabolites with antimicrobial activity. Phenolic compounds inhibit the colonization of Gammaproteobacteria [46]. Therefore, the epiphytic bacterial communities of submerged plants with different phenolic substrates are significantly different [16].
Submerged epiphytic bacterial communities play a crucial role in nutrient cycling in freshwater lakes. The results of our study indicate that bacterial genes associated with metabolic functions exhibit host plant specificity. Furthermore, the abundance of carbon cycling genes in M. verticillatum and P. lucens surpasses that observed in other epiphytic bacterial communities. It was concluded that the abundance of plant nutrient cycling–related genes correlates with specific leaf traits, noting that flat-leafed plants demonstrate superior photosynthesis compared to needle-leaf plants [47]. These findings align with Yu et al. who observed significant differences in the functions of epiphytic bacteria on three submerged macrophytes—H. verticillata, Vallisneria natans, and Potamogeton maackianus [10]. Discrepancies in the abundance of metabolic function genes in epiphytic bacteria may be attributed to their physicochemical properties. For example, during carbon cycle activity, Methylobacterium spp. (pink-pigmented facultative methylotrophic bacteria) rely on plant leaves to release compounds such as methane and methanol for growth and reproduction. These compounds contribute not only to the oxidative conversion of methane to carbon dioxide but also to the fixation of carbon dioxide by plants. The content of these nutrients on the leaf surface varies according to the plant species [15].
The abundance of genes involved in nitrate reduction was higher in the epiphytic bacteria of H. verticillata, and the abundance of genes related to organic nitrogen metabolism in M. verticillatum was higher than in other species. Nitrate assimilation/nitrogen transport, DNRA, ANRA, and denitrification have been standardized as nitrate reduction processes, primarily used for amino acid synthesis and promoting microbial metabolism and growth. The enrichment of genes related to the nitrate reducing pathway in the epiphytic bacteria of H. verticillata suggests that nitrogen depletion exceeds foreign nitrogen input or biological nitrogen fixation [48]. Jin et al. found that among five submerged aquatic plants, H. verticillata had the highest nitrogen removal capacity [49]. Choudhury et al. and Achouak et al. found lower denitrification rates in plants with allelopathy. Functional traits, such as plant biomass and leaf area, are also major factors affecting nitrogen accumulation and denitrification rates [50, 51]. However, this was not in accordance with our results, in which H. verticillata exhibited allelochemical effects and biomass and leaf area did not confer a significant advantage among the six plant species. Nitrogen cycling genes in H. verticillata were primarily contributed by members of the Comamonadaceae, which are common facultative denitrifying bacteria belonging to Betaproteobacteria [52]. We hypothesize that these strains play a crucial role in nitrogen degradation by H. verticillata and that diazotrophic communities and nitrozyme activities, such as those of Comamonadaceae, are significantly influenced by the host plant species [19]. This may explain the higher abundance of genes related to nitrate reduction in H. verticillata epiphytes. Phosphorus is an essential nutrient for plant growth, and inorganic phosphorus solubilization, organic phosphorus mineralization, and polyphosphate degradation are processes that convert phosphorus into an effective form for plant utilization. This allows plants to take up inorganic phosphorus, which microorganisms efficiently utilize and immobilize in the plant [53]. Massilia sp. was significantly enriched in the N. marina epiphytic bacterial community compared with other epiphytic bacterial communities. The positive contribution of phosphorus soluble genes (ppx-gppA, ppa, gcd, etc.) in Massilia sp. may explain the high expression of inorganic phosphorus process–related genes [54]. Genes attached to the polyphosphate degradation pathway, such as ppnk in M. verticillatum and spoT in C. demersum and H. verticillata, were enriched, suggesting that these plants play a substantial role in polyphosphate degradation, leading to the absorption of large amounts of inorganic phosphorus [55]. Pseudomonas, known for both accumulating and solubilizing phosphate, enriched in C. demersum, H. verticillata, and M. verticillatum, demonstrates a significant ability to degrade phosphate and performs well in polyphosphate degradation processes.
The composition of epiphytic bacterial communities is a crucial factor influencing the abundance of carbon, nitrogen, and phosphorus cycling genes [56], directly impacting ecosystem functioning [57]. Previous studies have highlighted that foliar nutrient sources regulate microbial growth, select and filter epiphytic bacterial communities, and contribute to differences in these communities on leaf surfaces [1]. For example, differences in the available sugar content on leaf surfaces limit bacterial community size [15], and leached metabolites such as polyphenols are selected for specific bacterial colonization [16]. In addition, leaf functional traits affect the abundance of nutrient cycling–related genes [47]. Different leaf shapes result in variations in light availability and other resources [37], providing additional microhabitats and ecological niches for epiphytic bacteria, thereby influencing their abundance and evenness [14].
Conclusion
Our findings reveal substantial variations in the abundance of epiphytic bacterial communities among six submerged plant species, while diversity and evenness remained statistically insignificant, and there was a significant correlation between plant diversity and plant complexity. Proteobacteria emerged as the predominant phylum, shared by the epiphytic bacterial communities on the leaf surfaces of all six submerged macrophytes. Noteworthy distinctions in the composition of epiphytic bacterial communities were observed among the plant species, spanning from the family to species taxonomic levels. Specifically, the epiphytic bacteria associated with submerged macrophytes, particularly those attached to M. verticillatum, assume a pivotal role in facilitating the transport and transformation of carbon, nitrogen, and phosphorus within aquatic ecosystems—a role strongly influenced by the host plant. The results of this study provide a deeper understanding of both the composition and functional aspects of epiphytic bacteria residing on submerged plant leaves, shedding light on their integral roles in freshwater ecosystems.
Data Availability
Data will be made available on request.
References
Schlechter RO, Miebach M, Remus-Emsermann MNP (2019) Driving factors of epiphytic bacterial communities: a review. J Adv Res 19:57–65
Eriksson P, Weisner S (1996) Functional differences in epiphytic microbial communities in nutrient-rich freshwater ecosystems: an assay of denitrifying capacity. Freshw Biol 36:555–562
He D, Zheng J, Ren L, Wu QL (2021) Substrate type and plant phenolics influence epiphytic bacterial assembly during short-term succession. Sci Total Environ 792:148410
Manirakiza B, Zhang S, Addo FG, Isabwe A, Nsabimana A (2022) Exploring microbial diversity and ecological function of epiphytic and surface sediment biofilm communities in a shallow tropical lake. Sci Total Environ 808:151821
Lachnit T, Meske D, Wahl M, Harder T, Schmitz R (2011) Epibacterial community patterns on marine macroalgae are host-specific but temporally variable. Environ Microbiol 13:655–665
Liu J, Sun X, Zuo Y, Hu Q, He X (2023) Plant species shape the bacterial communities on the phyllosphere in a hyper-arid desert. Microbiol Res 269:127314
Lu DC, Wang FQ, Amann RI, Teeling H, Du ZJ (2023) Epiphytic common core bacteria in the microbiomes of co-located green (Ulva), brown (Saccharina) and red (Grateloupia, Gelidium) macroalgae. Microbiome 11:126
Egan S, Harder T, Burke C, Steinberg P, Kjelleberg S, Thomas T (2013) The seaweed holobiont: understanding seaweed–bacteria interactions. FEMS Microbiol Rev 37:462–476
Burke C, Thomas T, Lewis M, Steinberg P, Kjelleberg S (2011) Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. ISME J 5:590–600
Yu W, Li J, Ma X, Lv T, Wang L, Li J, Liu C (2022) Community structure and function of epiphytic bacteria attached to three submerged macrophytes. Sci Total Environ 835:155546
Wijewardene L, Wu N, Fohrer N, Riis T (2022) Epiphytic biofilms in freshwater and interactions with macrophytes: current understanding and future directions. Aquat Bot 176:103467
Selvarajan R, Sibanda T, Venkatachalam S, Ogola HJO, Christopher Obieze C, Msagati TA (2019) Distribution, interaction and functional profiles of epiphytic bacterial communities from the rocky intertidal seaweeds, South Africa. Sci Rep 9:19835
Goldsborough L, McDougal R, North A (2005) Periphyton in freshwater lakes and wetlands in Periphyton: ecology, exploitation and management. CABI, Wallingford, UK, pp 71–89
Levi PS, Starnawski P, Poulsen B, Baattrup-Pedersen A, Schramm A, Riis T (2017) Microbial community diversity and composition varies with habitat characteristics and biofilm function in macrophyte-rich streams. Oikos. 126:398–409
Mercier J, Lindow SE (2000) Role of leaf surface sugars in colonization of plants by bacterial epiphytes. Appl Environ Microbiol 66:369–374
Hempel M, Blume M, Blindow I, Gross EM (2008) Epiphytic bacterial community composition on two common submerged macrophytes in brackish water and freshwater. BMC Microbiol 8:58
He D, Zheng J, Ren L (2012) Epiphytic bacterial communities on two common submerged macrophytes in Taihu Lake: diversity and host-specificity. Chin J Oceanol Limnol 30:237–247
Sun L, Wang J, Wu Y, Gao T, Liu C (2021) Community structure and function of epiphytic bacteria associated with Myriophyllum spicatum in Baiyangdian Lake, China. Front Microbiol 12:705509
Sun S, DeLuca TH, Zhang J, Wang G, Sun X, Hu Z, Wang W, Zhang W (2022) Evidence of endophytic nitrogen fixation as a potential mechanism supporting colonization of non-nodulating pioneer plants on a glacial foreland. Biol Fertil Soils 58:527–539
Yan L, Zhang S, Lin D, Guo C, Yan L, Wang S, He Z (2018) Nitrogen loading affects microbes, nitrifiers and denitrifiers attached to submerged macrophyte in constructed wetlands. Sci Total Environ 622-623:121–126
Unfried F, Becker S, Robb CS, Hehemann JH, Markert S, Heiden SE, Hinzke T, Becher D, Reintjes G, Krüger K, Avcı B, Kappelmann L, Hahnke RL, Fischer T, Harder J, Teeling H, Fuchs B, Barbeyron T, Amann RI, Schweder T (2018) Adaptive mechanisms that provide competitive advantages to marine bacteroidetes during microalgal blooms. Isme J 12:2894–2906
Shi L, Xia P, Lin T, Li G, Wang T, Xin D (2022) Temporal succession of bacterial community structure, co-occurrence patterns, and community assembly process in epiphytic biofilms of submerged plants in a Plateau Lake. Microb Ecol 85:87–99
Philonenko P, Postovalov S (2015) A new two-sample test for choosing between log-rank and Wilcoxon tests with right-censored data. J Stat Comput Simul 85:2761–2770
Liu D, Tong C (2017) Bacterial community diversity of traditional fermented vegetables in China. LWT. 86:40–48
Kozik AJ, Nakatsu CH, Chun H, Jones-Hall YL (2017) Age, sex, and TNF associated differences in the gut microbiota of mice and their impact on acute TNBS colitis. Exp Mol Pathol 103:311–319
Stewart TW, Shumaker TL, Radzio TA (2003) Linear and nonlinear effects of habitat structure on composition and abundance in the macroinvertebrate community of a large river. Am Midl Nat 149:293–305
Williamson MH, Lawton JH (1991) In: Bell SS, McCoy ED, Mushinsky HR (eds) Fractal geometry of ecological habitats in Habitat structure: the physical arrangement of objects in space. Springer, Netherlands, Dordrecht, pp 69–86
McAbendroth L, Ramsay PM, Foggo A, Rundle SD, Bilton DT (2005) Does macrophyte fractal complexity drive invertebrate diversity, biomass and body size distributions? Oikos. 111:279–290
Ferreiro N, Feijoó C, Giorgi A, Leggieri L (2011) Effects of macrophyte heterogeneity and food availability on structural parameters of the macroinvertebrate community in a Pampean stream. Hydrobiologia. 664:199–211
Warfe DM, Barmuta LA (2006) Habitat structural complexity mediates food web dynamics in a freshwater macrophyte community. Oecologia. 150:141–154
Ma J, Shi R, Han R, Ji M, Xu X, Wang G (2021) Community structure of epiphytic bacteria on Potamogeton pectinatus and the surrounding bacterioplankton in Hongze Lake. Mar Freshw Res 72:997–1003
Wu QL, Zwart G, Wu J, Kamst-van Agterveld MP, Liu S, Hahn MW (2007) Submersed macrophytes play a key role in structuring bacterioplankton community composition in the large, shallow, subtropical Taihu Lake, China. Environ Microbiol 9:2765–2774
Xia P, Yan D, Sun R, Song X, Lin T, Yi Y (2020) Community composition and correlations between bacteria and algae within epiphytic biofilms on submerged macrophytes in a plateau lake, southwest China. Sci Total Environ 727:138398
Shi L, Xia P, Lin T, Li G, Wang T, Du X (2022) Temporal succession of bacterial community structure, co-occurrence patterns, and community assembly process in epiphytic biofilms of submerged plants in a Plateau Lake. Microb Ecol 85:87–99
Cai X, Gao G, Tang X, Dong B, Dai J, Chen D, Song Y (2013) The response of epiphytic microbes to habitat and growth status of Potamogeton malaianus Miq. in Lake Taihu. J Basic Microbiol 53:828–837
Ercolani GL (1991) Distribution of epiphytic bacteria on olive leaves and the influence of leaf age and sampling time. Microb Ecol 21:35–48
Hao B, Wu H, Cao Y, Xing W, Jeppesen E, Li W (2017) Comparison of periphyton communities on natural and artificial macrophytes with contrasting morphological structures. Freshw Biol 62:1783–1793
Ferreiro N, Giorgi A, Feijoó C (2013) Effects of macrophyte architecture and leaf shape complexity on structural parameters of the epiphytic algal community in a Pampean stream. Aquat Ecol 47:389–401
Grossart H-P, Riemann L, Tang K (2013) Molecular and functional ecology of aquatic microbial symbionts. Front Microbiol 4:59
Nakai S, Inoue Y, Hosomi M, Murakami A (2000) Myriophyllum spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis aeruginosa. Water Res 34:3026–3032
Wium-Andersen S, Anthoni U, Christophersen C, Houen G (1982) Allelopathic effects on phytoplankton by substances isolated from aquatic macrophytes (Charales). Oikos. 39:187–190
Zhao B, Xing P, Wu QL (2021) Interactions between bacteria and fungi in macrophyte leaf litter decomposition. Environ Microbiol 23:1130–1144
Zhengqiang A, Xiaojian S, Xinyi C, Qi Z, Baohua G, Jin Z (2022) Diversity and network structure of epiphytic bacterial communities on different submerged macrophytes. J Lake Sci 34(4):1234–1249
Yadav RKP, Karamanoli K, Vokou D (2005) Bacterial colonization of the phyllosphere of Mediterranean perennial species as influenced by leaf structural and chemical features. Microb Ecol 50:185–196
Zhu Liping SD, Xiaohu L, Juwen H, Guangning L, Jingcheng X (2022) Research progress on allelopathic algae-inhibiting effects and action modes of submerged macrophytes. Appl Chem Industry 51:2337–2343
Zhao DY, Liu P, Fang C, Sun YM, Zeng J, Wang JQ, Ma T, Xiao YH, Wu QL (2013) Submerged macrophytes modify bacterial community composition in sediments in a large, shallow, freshwater lake. Can J Microbiol 59:237–244
Li J, Jin M, Neilson R, Hu S, Tang Y, Zhang Z, Huang F, Zhang J, Yang X (2023) Plant identity shapes phyllosphere microbiome structure and abundance of genes involved in nutrient cycling. Sci Total Environ 865:161245
Zhu YG, Peng J, Chen C, Xiong C, Li S, Ge A, Wang E, Liesack W (2023) Harnessing biological nitrogen fixation in plant leaves. Trends Plant Sci 28(12):1391–1405
Jin S, Zhou J, Bao W, Chen J, Li D, Li Y (2017) Comparison of nitrogen and phosphorus uptake and water purification abilityof five submerged macrophytes. Environ Sci 38:156–161
Choudhury MI, McKie BG, Hallin S, Ecke F (2018) Mixtures of macrophyte growth forms promote nitrogen cycling in wetlands. Sci Total Environ 635:1436–1443
Achouak W, Abrouk D, Guyonnet J, Barakat M, Ortet P, Simon L, Lerondelle C, Heulin T, Haichar FEZ (2019) Plant hosts control microbial denitrification activity. FEMS Microbiol Ecol 95:fiz021
Liu J, Yu S, Cong D, Yue J, Yang C, Wang X, Ni C, Wang T (2021) Optimization of a novel single air-lift sequencing bioreactor for raw piggery wastewater treatment: nutrients removal and microbial community structure analysis. Bioresour Technol 321:124431
Dai Z, Liu G, Chen H, Chen C, Wang J, Ai S, Wei D, Li D, Ma B, Tang C, Brookes PC, Xu J (2020) Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J 14:757–770
Samaddar S, Chatterjee P, Truu J, Anandham R, Kim S, Sa T (2019) Long-term phosphorus limitation changes the bacterial community structure and functioning in paddy soils. Appl Soil Ecol 134:111–115
Chekwube EM, Oluranti BO (2021) The influence of soil fertilization on the distribution and diversity of phosphorus cycling genes and microbes community of maize rhizosphere using shotgun metagenomics. Genes. 12:1022–1022
Xiang Q, Chen Q-L, Zhu D, Yang X-R, Qiao M, Hu H-W, Zhu Y-G (2020) Microbial functional traits in phyllosphere are more sensitive to anthropogenic disturbance than in soil. Environ Pollut 265:114954
Laforest Lapointe I, Paquette A, Messier C, Kembel SW (2017) Leaf bacterial diversity mediates plant diversity and ecosystem function relationships. Nature. 546:145–147
Acknowledgements
We would like to thank the editor and reviewers who helped to improve the manuscript.
Funding
This research was funded by the National Nature Science Foundation of China (32360288) and the Guizhou Provincial Key Technology R&D Program (No. 2023216).
Author information
Authors and Affiliations
Contributions
Xin Wang: software, investigation, data curation, writing — original draft. YI Liu: resources, writing — review and editing. Chun Qing: writing — review and editing. Pinhua Xia: conceptualization, methodology, writing — original draft. Jin Zeng: resources, writing — review and editing. Jixing Dong: investigation, data curation.
Corresponding author
Ethics declarations
Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, X., Liu, Y., Qing, C. et al. Analysis of diversity and function of epiphytic bacterial communities associated with macrophytes using a metagenomic approach. Microb Ecol 87, 37 (2024). https://doi.org/10.1007/s00248-024-02346-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00248-024-02346-7