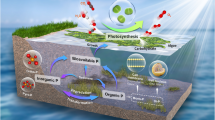
Abstract
Heterotrophic prokaryotes (HP) contribute largely to dissolved organic matter (DOM) processing in the ocean, but they also release diverse organic substances. The bioavailability of DOM released by HP under varying environmental conditions has not been fully elucidated. In this study, we investigated the bioavailability of DOM released by a single bacterial strain (Sphingopyxis alaskensis) and 2 natural HP communities grown under P-replete and P-limited conditions. The released DOM (HP-DOM) was used as a substrate for natural HP communities at a coastal site in the Northwestern Mediterranean Sea. We followed changes in HP growth, enzymatic activity, diversity, and community composition together with the consumption of HP-DOM fluorescence (FDOM). HP-DOM produced under P-replete and P-limited conditions promoted significant growth in all incubations. No clear differences in HP-DOM lability released under P-repletion and P-limitation were evidenced based on the HP growth, and P-limitation was not demonstrated to decrease HP-DOM lability. However, HP-DOM supported the growth of diverse HP communities, and P-driven differences in HP-DOM quality were selected for different indicator taxa in the degrading communities. The humic-like fluorescence, commonly considered recalcitrant, was consumed during the incubations when this peak was initially dominating the FDOM pool, and this consumption coincided with higher alkaline phosphatase activity. Taken together, our findings emphasize that HP-DOM lability is dependent on both DOM quality, which is shaped by P availability, and the composition of the consumer community.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon request. Sequence data will be uploaded to the EMBL-ENA data repository.
References
Hansell DA (2013) Recalcitrant dissolved organic carbon fractions. Ann Rev Mar Sci 5:421–445. https://doi.org/10.1146/annurev-marine-120710-100757
Azam F, Fenchel T, Field J et al (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10:257–263. https://doi.org/10.3354/meps010257
Jiao N, Herndl GJ, Hansell DA et al (2010) Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nat Rev Microbiol 8:593–599. https://doi.org/10.1038/nrmicro2386
Legendre L, Rivkin RB, Weinbauer MG et al (2015) The microbial carbon pump concept: Potential biogeochemical significance in the globally changing ocean. Prog Oceanogr 134:432–450. https://doi.org/10.1016/j.pocean.2015.01.008
Jiao N, Azam F (2011) Microbial carbon pump and its significance for carbon sequestration in the ocean. Microbial carbon pump in the ocean. Science/AAAS, Washington, DC
Daoud ABA, Tremblay L (2019) HPLC-SEC-FTIR characterization of the dissolved organic matter produced by the microbial carbon pump. Mar Chem 215:103668. https://doi.org/10.1016/j.marchem.2019.103668
LaBrie R, Péquin B, Fortin St-Gelais N et al (2022) Deep ocean microbial communities produce more stable dissolved organic matter through the succession of rare prokaryotes. Sci Adv 8:eabn0035. https://doi.org/10.1126/sciadv.abn0035
Lechtenfeld OJ, Hertkorn N, Shen Y et al (2015) Marine sequestration of carbon in bacterial metabolites. Nat Commun 6:6711. https://doi.org/10.1038/ncomms7711
Ogawa H (2001) Production of refractory dissolved organic matter by bacteria. Science 292:917–920. https://doi.org/10.1126/science.1057627
Ortega-Retuerta E, Devresse Q, Caparros J et al (2021) Dissolved organic matter released by two marine heterotrophic bacterial strains and its bioavailability for natural prokaryotic communities. Environ Microbiol 23:1363–1378. https://doi.org/10.1111/1462-2920.15306
Osterholz H, Niggemann J, Giebel H-A et al (2015) Inefficient microbial production of refractory dissolved organic matter in the ocean. Nat Commun 6:7422. https://doi.org/10.1038/ncomms8422
Jiao N, Robinson C, Azam F et al (2014) Mechanisms of microbial carbon sequestration in the ocean – future research directions. Biogeosciences 11:5285–5306. https://doi.org/10.5194/bg-11-5285-2014
Alonso-Sáez L, Gasol JM (2007) Seasonal variations in the contributions of different bacterial groups to the uptake of low-molecular-weight compounds in Northwestern Mediterranean coastal waters. Appl Environ Microbiol 73:3528–3535. https://doi.org/10.1128/AEM.02627-06
Krüger K, Chafee M, Ben Francis T et al (2019) In marine Bacteroidetes the bulk of glycan degradation during algae blooms is mediated by few clades using a restricted set of genes. ISME J 13:2800–2816. https://doi.org/10.1038/s41396-019-0476-y
McCarren J, Becker JW, Repeta DJ et al (2010) Microbial community transcriptomes reveal microbes and metabolic pathways associated with dissolved organic matter turnover in the sea. Proc Natl Acad Sci U S A 107:16420–16427. https://doi.org/10.1073/pnas.1010732107
Sala MM, Ruiz-González C, Borrull E et al (2020) Prokaryotic capability to use organic substrates across the global tropical and subtropical ocean. Front Microbiol 11
Arnosti C, Steen AD, Ziervogel K et al (2011) Latitudinal gradients in degradation of marine dissolved organic carbon. PloS One 6:e28900. https://doi.org/10.1371/journal.pone.0028900
Lønborg C, Álvarez-Salgado XA, Letscher RT, Hansell DA (2018) Large stimulation of recalcitrant dissolved organic carbon degradation by increasing ocean temperatures. Front Mar Sci 4:436. https://doi.org/10.3389/fmars.2017.00436
Obernosterer I, Kawasaki N, Benner R (2003) P-limitation of respiration in the Sargasso Sea and uncoupling of bacteria from P-regeneration in size-fractionation experiments. Aquat Microb Ecol 32:229–237. https://doi.org/10.3354/ame032229
Lazzari P, Solidoro C, Salon S, Bolzon G (2016) Spatial variability of phosphate and nitrate in the Mediterranean Sea: a modeling approach. Deep-Sea Res I Oceanogr Res Pap 108:39–52. https://doi.org/10.1016/j.dsr.2015.12.006
Thingstad TF, Zweifel UL, Rassoulzadegan F (1998) P limitation of heterotrophic bacteria and phytoplankton in the northwest Mediterranean. Limnol Oceanogr 43:88–94. https://doi.org/10.4319/lo.1998.43.1.0088
Pinhassi J, Gómez-Consarnau L, Alonso-Sáez L et al (2006) Seasonal changes in bacterioplankton nutrient limitation and their effects on bacterial community composition in the NW Mediterranean Sea. Aquat Microb Ecol 44:241–252. https://doi.org/10.3354/ame044241
Obernosterer I, Herndl G (1995) Phytoplankton extracellular release and bacterial growth: dependence on the inorganic N: P ratio. Mar Ecol Prog Ser 116:247–257. https://doi.org/10.3354/meps116247
Puddu A, Zoppini A, Fazi S et al (2003) Bacterial uptake of DOM released from P-limited phytoplankton. FEMS Microbiol Ecol 46:257–268. https://doi.org/10.1016/S0168-6496(03)00197-1
Romano S, Dittmar T, Bondarev V et al (2014) Exo-metabolome of Pseudovibrio sp. FO-BEG1 analyzed by ultra-high resolution mass spectrometry and the effect of phosphate limitation. PloS One 9:e96038. https://doi.org/10.1371/journal.pone.0096038
Bouchachi N, Obernosterer I, Marie B et al (2022) Phosphorus limitation determines the quality of dissolved organic matter released by marine heterotrophic prokaryotes. Limnol Oceanogr Lett n/a. https://doi.org/10.1002/lol2.10287
Thompson SK, Cotner JB (2020) P-limitation drives changes in DOM production by aquatic bacteria. Aquat Microb Ecol 85:35–46. https://doi.org/10.3354/ame01940
Coble PG, Green SA, Blough NV, Gagosian RB (1990) Characterization of dissolved organic matter in the Black Sea by fluorescence spectroscopy. Nature 348:432–435. https://doi.org/10.1038/348432a0
Yamashita Y, Tanoue E (2008) Production of bio-refractory fluorescent dissolved organic matter in the ocean interior. Nat Geosci 1:579–582. https://doi.org/10.1038/ngeo279
Catalá TS, Reche I, Fuentes-Lema A et al (2015) Turnover time of fluorescent dissolved organic matter in the dark global ocean. Nat Commun 6:5986. https://doi.org/10.1038/ncomms6986
Jiao N, Cai R, Zheng Q et al (2018) Unveiling the enigma of refractory carbon in the ocean. Natl Sci Rev 5:459–463. https://doi.org/10.1093/nsr/nwy020
Lønborg C, Álvarez-Salgado XA, Davidson K, Miller AEJ (2009) Production of bioavailable and refractory dissolved organic matter by coastal heterotrophic microbial populations. Estuar Coast Shelf Sci 82:682–688. https://doi.org/10.1016/j.ecss.2009.02.026
Nieto-Cid M, Álvarez-Salgado XA, Pérez FF (2006) Microbial and photochemical reactivity of fluorescent dissolved organic matter in a coastal upwelling system. Limnol Oceanogr 51:1391–1400. https://doi.org/10.4319/lo.2006.51.3.1391
Yamashita Y, Tanoue E (2003) Chemical characterization of protein-like fluorophores in DOM in relation to aromatic amino acids. Mar Chem 82:255–271. https://doi.org/10.1016/S0304-4203(03)00073-2
Lebaron P, Parthuisot N, Catala P (1998) Comparison of blue nucleic acid dyes for flow cytometric enumeration of bacteria in aquatic systems. Appl Environ Microbiol 64:1725–1730
Obernosterer I, Catala P, Lami R et al (2008) Biochemical characteristics and bacterial community structure of the sea surface microlayer in the South Pacific Ocean. Biogeosciences 5:693–705
Parada AE, Needham DM, Fuhrman JA (2016) Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol 18:1403–1414. https://doi.org/10.1111/1462-2920.13023
Callahan BJ, McMurdie PJ, Rosen MJ et al (2016) DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Parks DH, Chuvochina M, Waite DW et al (2018) A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol 36:996–1004. https://doi.org/10.1038/nbt.4229
Kassambara A (2021) rstatix: Pipe-Friendly Framework for Basic Statistical Tests
Oksanen J, Blanchet FG, Friendly M, et al (2020) vegan community ecology package version 2.5–7. November 2020
Roberts DW (2019) labdsv: Ordination and Multivariate Analysis for Ecology
Nieto-Cid M, Álvarez-Salgado XA, Gago J, Pérez FF (2005) DOM fluorescence, a tracer for biogeochemical processes in a coastal upwelling system (NW Iberian Peninsula). Mar Ecol Prog Ser 297:33–50. https://doi.org/10.3354/meps297033
Romera-Castillo C, Sarmento H, Álvarez-Salgado XA et al (2011) Net production and consumption of fluorescent colored dissolved organic matter by natural bacterial assemblages growing on marine phytoplankton exudates. Appl Environ Microbiol 77:7490–7498. https://doi.org/10.1128/AEM.00200-11
Cory RM, Kaplan LA (2012) Biological lability of streamwater fluorescent dissolved organic matter. Limnol Oceanogr 57:1347–1360. https://doi.org/10.4319/lo.2012.57.5.1347
Varela MM, Rodríguez-Ramos T, Guerrero-Feijóo E, Nieto-Cid M (2020) Changes in activity and community composition shape bacterial responses to size-fractionated marine DOM. Front Microbiol 11:586148. https://doi.org/10.3389/fmicb.2020.586148
Arai K, Wada S, Shimotori K et al (2018) Production and degradation of fluorescent dissolved organic matter derived from bacteria. J Oceanogr 74:39–52. https://doi.org/10.1007/s10872-017-0436-y
Xie R, Wang Y, Chen Q et al (2020) Coupling between carbon and nitrogen metabolic processes mediated by coastal microbes in Synechococcus-derived organic matter addition incubations. Front Microbiol 11
Lidbury IDEA, Scanlan DJ, Murphy ARJ et al (2022) A widely distributed phosphate-insensitive phosphatase presents a route for rapid organophosphorus remineralization in the biosphere. Proc Natl Acad Sci U S A 119:e2118122119. https://doi.org/10.1073/pnas.2118122119
Noriega-Ortega BE, Wienhausen G, Mentges A et al (2019) Does the chemodiversity of bacterial exometabolomes sustain the chemodiversity of marine dissolved organic matter? Front Microbiol 10. https://doi.org/10.3389/fmicb.2019.00215
Landa M, Cottrell MT, Kirchman DL et al (2014) Phylogenetic and structural response of heterotrophic bacteria to dissolved organic matter of different chemical composition in a continuous culture study. Environ Microbiol 16:1668–1681. https://doi.org/10.1111/1462-2920.12242
Müller O, Seuthe L, Bratbak G, Paulsen ML (2018) Bacterial response to permafrost derived organic matter input in an Arctic Fjord. Front Mar Sci 5
Nelson CE, Carlson CA (2012) Tracking differential incorporation of dissolved organic carbon types among diverse lineages of Sargasso Sea bacterioplankton. Environ Microbiol 14:1500–1516. https://doi.org/10.1111/j.1462-2920.2012.02738.x
Luo H, Benner R, Long RA, Hu J (2009) Subcellular localization of marine bacterial alkaline phosphatases. Proc Natl Acad Sci 106:21219–21223. https://doi.org/10.1073/pnas.0907586106
Xiao X, Guo W, Li X et al Viral lysis alters the optical properties and biological availability of dissolved organic matter derived from Prochlorococcus Picocyanobacteria. Appl Environ Microbiol 87:e02271–20. https://doi.org/10.1128/AEM.02271-20
Acknowledgements
This work was supported by the Caramba (European Commission, H2020-MSCA-IF-2015-703991), ODISEA (Institut National des Sciences de l’Univers LEFE/CYBER 2019), and MicroPump (Agence Nationale de Recherche ANR-20-CE01-0007) projects to EOR. Flow cytometric analyses were performed at the BioPic cytometry and imaging platform (Sorbonne University/CNRS). The authors thank the crew of R/V “Nereis II” and the technicians of the Banyuls observation service for their assistance in getting Mediterranean Sea samples for the natural communities’ isolation.
Funding
This work was supported by the Caramba (European commission, H2020-MSCA-IF-2015-703991), ODISEA (INSU LEFE/CYBER 2019), and MicroPump (ANR JCJC 2020) projects to EOR.
Author information
Authors and Affiliations
Contributions
NB, EOR, and IO conceived the experimental work. NB, FL, CCB, LS, and EOR performed the experiments, with active contributions from OC, BM, and PC to chemical and biological analyses. NB analyzed the data with contributions from FL, CCB, LS, and EOR. NB wrote the manuscript with significant contributions from IO and EOR. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Supplementary information
ESM 1
(DOCX 79068 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bouchachi, N., Obernosterer, I., Carpaneto Bastos, C. et al. Effects of Phosphorus Limitation on the Bioavailability of DOM Released by Marine Heterotrophic Prokaryotes. Microb Ecol 86, 1961–1971 (2023). https://doi.org/10.1007/s00248-023-02201-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-023-02201-1