Abstract
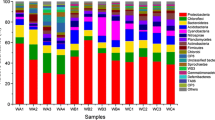
In this study, we report the characteristics of a microbial community in sampled groundwater and elucidate the effects of temperature and pH disturbances on bacterial structure and nitrogen-cycling functions. The predominant phyla of candidate OD1, candidate OP3, and Proteobacteria represented more than half of the total bacteria, which clearly manifested as a “low nucleic acid content (LNA) bacteria majority” type via flow cytometric fingerprint. The results showed that LNA bacteria were more tolerant to rapid changes in temperature and pH, compared to high nucleic acid content (HNA) bacteria. A continuous temperature increase test demonstrated that the LNA bacterial group was less competitive than the HNA bacterial group in terms of maintaining their cell intactness and growth potential. In contrast, the percentage of intact LNA bacteria was maintained at nearly 70% with pH decrease, despite a 50% decrease in total intact cells. Next-generation sequencing results revealed strong resistance and growth potential of phylum Proteobacteria when the temperature increased or the pH decreased in groundwater, especially for subclasses α-, β-, and γ-Proteobacteria. In addition, relative abundance of nitrogen-related functional genes by qPCR showed no difference in nitrifiers or denitrifiers within 0.45 μm-captured and 0.45 μm-filterable bacteria due to phylogenetic diversity. One exception was the monophyletic anammox bacteria that belong to the phylum Planctomycetes, which were mostly captured on a 0.45-μm filter. Furthermore, we showed that both temperature increase and pH decrease could enhance the denitrification potential, whereas the nitrification and anammox potentials were weakened.








Similar content being viewed by others
References
Braun B, Schroder J, Knecht H, Szewzyk U (2016) Unraveling the microbial community of a cold groundwater catchment system. Water Res 107:113–126. https://doi.org/10.1016/j.watres.2016.10.040
Besmer MD, Epting J, Page RM, Sigrist JA, Huggenberger P, Hammes F (2016) Online flow cytometry reveals microbial dynamics influenced by concurrent natural and operational events in groundwater used for drinking water treatment. Sci Rep 6:38462. https://doi.org/10.1038/srep38462
Tufenkji N, Ryan JN, Elimelech M (2002) The promise of bank filtration. Environ Sci Technol36: 422A
Griebler C, Lueders T (2010) Microbial biodiversity in groundwater ecosystems. Freshw Biol 54:649–677 https://doi.org/j.1365-2427.2008.02013.x
Bouvier T, Del Giorgio PA, Gasol JM (2007) A comparative study of the cytometric characteristics of high and low nucleic-acid bacterioplankton cells from different aquatic ecosystems. Environ Microbiol 9:2050–2066. https://doi.org/10.1111/j.1462-2920.2007.01321.x
Wang Y, Hammes F, Boon N, Chami M, Egli T (2009) Isolation and characterization of low nucleic acid (LNA)-content bacteria. ISME J 3:889–902. https://doi.org/10.1038/ismej.2009.46
Luef B, Frischkorn KR, Wrighton KC, Holman HY, Birarda G, Thomas BC, Singh A, Williams KH, Siegerist CE, Tringe SG, Downing KH, Comolli LR, Banfield JF (2015) Diverse uncultivated ultra-small bacterial cells in groundwater. Nat Commun 6:6372. https://doi.org/10.1038/ncomms7372
Wang Y, Hammes F, Düggelin M, Egli T (2008) Influence of size, shape, and flexibility on bacterial passage through micropore membrane filters. Environ Sci Technol 42:6749–6754. https://doi.org/10.1021/es800720n
Miteva VI, Brenchley JE (2005) Detection and isolation of ultrasmall microorganisms from a 120,000-year-old Greenland glacier ice core. Appl Environ Microbiol 71:7806–7818. https://doi.org/10.1128/AEM.71.12.7806-7818.2005
Proctor CR, Besmer MD, Langenegger T, Beck K, Walser JC, Ackermann M, Burgmann H, Hammes F (2018) Phylogenetic clustering of small low nucleic acid-content bacteria across diverse freshwater ecosystems. ISME J 12:1344–1359. https://doi.org/10.1038/s41396-018-0070-8
Ramseier MK, von Gunten U, Freihofer P, Hammes F (2011) Kinetics of membrane damage to high (HNA) and low (LNA) nucleic acid bacterial clusters in drinking water by ozone, chlorine, chlorine dioxide, monochloramine, ferrate (VI), and permanganate. Water Res 45:1490–1500. https://doi.org/10.1016/j.watres.2010.11.016
Laidlaw PP, Elford WJ (1936) A new group of filterable organisms. P Roy Soc B-Biol Sci 120:292–303
Huete-Stauffer TM, Arandia-Gorostidi N, Alonso-Saez L, Moran XA (2016) Experimental warming decreases the average size and nucleic acid content of marine bacterial communities. Front Microbiol 7:730. https://doi.org/10.3389/fmicb.2016.00730
Hessen DO, Daufresne M, Leinaas HP (2013) Temperature-size relations from the cellular-genomic perspective. Biol Rev Camb Philos Soc 88:476–489. https://doi.org/10.1111/brv.12006
Sheridan JA, Bickford D (2011) Shrinking body size as an ecological response to climate change. Nat Clim Chang 1:401–406. https://doi.org/10.1038/nclimate1259
Daufresne M, Lengfellner K, Sommer U (2009) Global warming benefits the small in aquatic ecosystems. PNAS 106:12788–12793. https://doi.org/10.1073/pnas.0902080106
Gardner JL, Peters A, Kearney MR, Joseph L, Heinsohn R (2011) Declining body size: a third universal response to warming? Trends Ecol Evol 26:285–291. https://doi.org/10.1016/j.tree.2011.03.005
White EP, Ernest SK, Kerkhoff AJ, Enquist BJ (2007) Relationships between body size and abundance in ecology. Trends Ecol Evol 22:323–330. https://doi.org/10.1016/j.tree.2007.03.007
Sabath N, Ferrada E, Barve A, Wagner A (2013) Growth temperature and genome size in bacteria are negatively correlated, suggesting genomic streamlining during thermal adaptation. Genome Biol Evol 5:966–977. https://doi.org/10.1093/gbe/evt050
Visser ME, Both C (2005) Shifts in phenology due to global climate change: the need for a yardstick. P Roy Soc B-Biol Sci 272:2561–2569. https://doi.org/10.1098/rspb.2005.3356
Meron D, Atias E, Iasur Kruh L, Elifantz H, Minz D, Fine M, Banin E (2011) The impact of reduced pH on the microbial community of the coral Acropora eurystoma. ISME J 5:51–60. https://doi.org/10.1038/ismej.2010.102
Yanagawa K, Morono Y, de Beer D, Haeckel M, Sunamura M, Futagami T, Hoshino T, Terada T, Nakamura K, Urabe T, Rehder G, Boetius A, Inagaki F (2013) Metabolically active microbial communities in marine sediment under high-CO(2) and low-pH extremes. ISME J 7:555–567. https://doi.org/10.1038/ismej.2012.124
Fine M, Tchernov D (2007) Scleractinian coral species survive and recover from decalcification. Science 315:1811–1811. https://doi.org/10.1126/science.1137094
Crawley A, Kline DI, Dunn S, Anthony K, Dove S (2010) The effect of ocean acidification on symbiont photorespiration and productivity in Acropora formosa. Glob Chang Biol 16:851–863. https://doi.org/10.1111/j.1365-2486.2009.01943.x
Vega TR, Willnerhall D, Rodriguezmueller B, Desnues C, Edwards RA, Angly F, Dinsdale E, Kelly L, Rohwer F (2009) Metagenomic analysis of stressed coral holobionts. Environ Microbiol 11:2148–2163. https://doi.org/10.1111/j.1462-2920.2009.01935.x
Liu F, Sun J, Wang J, Zhang Y (2017) Groundwater acidification in shallow aquifers in Pearl River Delta, China: distribution, factors, and effects. Geochem J 51:373–384. https://doi.org/10.2343/geochemj.2.0476
Highton MP, Roosa S, Crawshaw J, Schallenberg M, Morales SE (2016) Physical factors correlate to microbial community structure and nitrogen cycling gene abundance in a nitrate fed eutrophic lagoon. Front Microbiol 7:1691. https://doi.org/10.3389/fmicb.2016.01691
Tilman D (1999) Global environmental impacts of agricultural expansion: the need for sustainable and efficient practices. PNAS 96:5995–6000
Hu Y, He F, Ma L, Zhang Y, Wu Z (2016) Microbial nitrogen removal pathways in integrated vertical-flow constructed wetland systems. Bioresour Technol 207:339–345. https://doi.org/10.1016/j.biortech.2016.01.106
Zeng J, Lou K, Zhang CJ, Wang JT, Hu HW, Shen JP, Zhang LM, Han LL, Zhang T, Lin Q, Chalk PM, He JZ (2016) Primary succession of nitrogen cycling microbial communities along the deglaciated forelands of Tianshan Mountain, China. Front Microbiol 7:1353. https://doi.org/10.3389/fmicb.2016.01353
Hammes F, Egli T (2005) New method for assimilable organic carbon determination using flow-cytometric enumeration and a natural microbial consortium as inoculum. Environ Sci Technol 39:3289–3294. https://doi.org/10.1021/es048277c
Liu X, Wang J, Liu T, Kong W, He X, Jin Y, Zhang B (2015) Effects of assimilable organic carbon and free chlorine on bacterial growth in drinking water. PLoS One 10: e0128825
Vital M, Hammes F, Egli T (2012) Competition of Escherichia coli O157 with a drinking water bacterial community at low nutrient concentrations. Water Res 46:6279–6290. https://doi.org/10.1016/j.watres.2012.08.043
Berney M, Vital M, Hülshoff I, Weilenmann HU, Egli T, Hammes F (2008) Rapid, cultivation-independent assessment of microbial viability in drinking water. Water Res 42:4010–4018. https://doi.org/10.1016/j.watres.2008.07.017
Hammes F, Goldschmidt F, Vital M, Wang Y, Egli T (2010) Measurement and interpretation of microbial adenosine tri-phosphate (ATP) in aquatic environments. Water Res 44:3915–3923. https://doi.org/10.1016/j.watres.2010.04.015
Liu J, Hua ZS, Chen LX, Kuang JL, Li SJ, Shu WS, Huang LN (2014) Correlating microbial diversity patterns with geochemistry in an extreme and heterogeneous environment of mine tailings. Appl Environ Microbiol 80:3677–3686. https://doi.org/10.1128/AEM.00294-14
Liu Z, Lozupone C, Hamady M, Bushman FD, Knight R (2007) Short pyrosequencing reads suffice for accurate microbial community analysis. Nucleic Acids Res 35:e120. https://doi.org/10.1093/nar/gkm541
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. https://doi.org/10.1038/nmeth.2604
Tsushima I, Kindaichi T, Okabe S (2007) Quantification of anaerobic ammonium-oxidizing bacteria in enrichment cultures by real-time PCR. Water Res 41:785–794. https://doi.org/10.1016/j.watres.2006.11.024
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Attard E, Poly F, Commeaux C, Laurent F, Terada A, Smets BF, Recous S, Roux XL (2010) Shifts between Nitrospira- and Nitrobacter-like nitrite oxidizers underlie the response of soil potential nitrite oxidation to changes in tillage practices. Environ Microbiol 12:315–326. https://doi.org/10.1111/j.1462-2920.2009.02070.x
Henry S, Baudoin E, López-Gutiérrez JC, Martin-Laurent F, Brauman A, Philippot L (2005) Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J Microbiol Methods 61:289–290. https://doi.org/10.1016/j.mimet.2004.07.002
Kandeler E, Deiglmayr K, Tscherko D, Bru D, Philippot L (2006) Abundance of narG, nirS, nirK, and nosZ genes of denitrifying bacteria during primary successions of a glacier foreland. Appl Environ Microbiol 72:5957–5962. https://doi.org/10.1128/AEM.00439-06
Henry S, Bru D, Stres B, Hallet S, Philippot L (2006) Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl Environ Microbiol 72:5181–5189. https://doi.org/10.1128/AEM.00231-06
Hartman AL, Lough DM, Barupal DK, Fiehn O, Fishbein T, Zasloff M, Eisen JA, Gordon JI (2009) Human gut microbiome adopts an alternative state following small bowel transplantation. PNAS 106:17187–17192. https://doi.org/10.1073/pnas.0904847106
Wang Y, Hammes F, Boon N, Egli T (2007) Quantification of the filterability of freshwater bacteria through 0.45, 0.22, and 0.1 m pore size filters and shape-dependent enrichment of filterable bacterial communities. Environ Sci Technol 41:7080–7086. https://doi.org/10.1021/es0707198
Morita RY (1997) Bacteria in oligotrophic environments: starvation-survival lifestyle. Chapman & Hall, New York, pp 50–89
Kantor RS, Wrighton KC, Handley KM, Sharon I, Hug LA, Castelle CJ, Thomas BC, Banfield JF (2013) Small genomes and sparse metabolisms of sediment-associated bacteria from four candidate phyla. Mbio 4:00708–00713. https://doi.org/10.1128/mBio.00708-13
Shin NR, Whon TW, Bae JW (2015) Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33:496–503. https://doi.org/10.1016/j.tibtech.2015.06.011
Hahn MW (2004) Broad diversity of viable bacteria in ‘sterile’ (0.2 microm) filtered water. Res Microbiol 155:688–691. https://doi.org/10.1016/j.resmic.2004.05.003
Haller CM, Rölleke S, Vybiral D, Witte A, Velimirov B (2000) Investigation of 0.2 μm filterable bacteria from the Western Mediterranean Sea using a molecular approach: dominance of potential starvation forms. FEMS Microbiol Ecol 31:153–161. https://doi.org/10.1016/S0168-6496(99)00096-3
Vybiral D, Denner EBM, Haller CM, Busse HJ, Witte A, Höfle MG, Velimirov B (1999) Polyphasic classification of 0.2 μm filterable bacteria from the Western Mediterranean Sea. Syst Appl Microbiol 22:635–646. https://doi.org/10.1016/S0723-2020(99)80017-4
Nimbkar N, Rajvanshi AK (2013) Simple filtration and low-temperature sterilization of drinking water. Curr Sci 104:519–522
Spinks AT, Dunstan RH, Harrison T, Coombes P, Kuczera G (2006) Thermal inactivation of water-borne pathogenic and indicator bacteria at sub-boiling temperatures. Water Res 40:1326–1332. https://doi.org/10.1016/j.watres.2006.01.032
Ciochetti DA, Metcalf RH (1984) Pasteurization of naturally contaminated water with solar energy. Appl Environ Microbiol 47:223–228
Reineke K, Mathys A, Heinz V, Knorr D (2013) Mechanisms of endospore inactivation under high pressure. Trends Microbiol 21:296–304. https://doi.org/10.1016/j.tim.2013.03.001
Aüllo T, Ranchoupeyruse A, Ollivier B, Magot M (2013) Desulfotomaculum spp. and related gram-positive sulfate-reducing bacteria in deep subsurface environments. Front Microbiol 4: 362. https://doi.org/10.3389/fmicb.2013.00362
Hassani M, Mañas P, Raso J, Condón S, Pagán R (2005) Predicting heat inactivation of listeria monocytogenes under nonisothermal treatments. J Food Prot 68:736–743. https://doi.org/10.4315/0362-028X-68.4.736
Kim KT, Murano EA, Olson DG (2010) Heating and storage conditions affect survival and recovery of listeria monocytogenes in ground pork. J Food Sci 59:30–32. https://doi.org/10.1111/j.1365-2621.1994.tb06890.x
Eydal HS, Pedersen K (2007) Use of an ATP assay to determine viable microbial biomass in Fennoscandian Shield groundwater from depths of 3-1000 m. J Microbiol Methods 70:363–373. https://doi.org/10.1016/j.mimet.2007.05.012
Nescerecka A, Juhna T, Hammes F (2016) Behavior and stability of adenosine triphosphate (ATP) during chlorine disinfection. Water Res 101:490–497. https://doi.org/10.1016/j.watres.2016.05.087
Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S (2011) A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75:14–49. https://doi.org/10.1128/MMBR.00028-10
Najar IN, Sherpa MT, Das S, Das S, Thakur N (2018) Microbial ecology of two hot springs of Sikkim: predominate population and geochemistry. Sci Total Environ 637-638:730–745. https://doi.org/10.1016/j.scitotenv.2018.05.037
Keshri J, Pradeep Ram AS, Nana PA, Sime-Ngando T (2018) Taxonomical resolution and distribution of bacterioplankton along the vertical gradient reveals pronounced spatiotemporal patterns in contrasted temperate freshwater lakes. Microb Ecol 76:372–386. https://doi.org/10.1007/s00248-018-1143-y
Ghosh A, Bhadury P (2018). Exploring biogeographic patterns of bacterioplankton communities across global estuaries. Microbiologyopen:e741. https://doi.org/10.1002/mbo3.741
Jin D, Kong X, Cui B, Jin S, Xie Y, Wang X, Deng Y (2018) Bacterial communities and potential waterborne pathogens within the typical urban surface waters. Sci Rep 8:13368. https://doi.org/10.1038/s41598-018-31706-w
Salmaso N, Albanese D, Capelli C, Boscaini A, Pindo M, Donati C (2018) Diversity and cyclical seasonal transitions in the bacterial community in a large and deep perialpine lake. Microb Ecol 76:125–143. https://doi.org/10.1007/s00248-017-1120-x
Amin A, Ahmed I, Salam N, Kim BY, Singh D, Zhi XY, Xiao M, Li WJ (2017) Diversity and distribution of thermophilic bacteria in hot springs of Pakistan. Microb Ecol 74:1–12. https://doi.org/10.1007/s00248-017-0930-1
López-López O, Knapik K, Cerdán ME, González-Siso MI (2015) Metagenomics of an alkaline hot spring in Galicia (Spain): microbial diversity analysis and screening for novel lipolytic enzymes. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.01291
Liu K, Liu Y, Jiao N, Xu B, Gu Z, Xing T, Xiong J (2017) Bacterial community composition and diversity in Kalakuli, an alpine glacial-fed lake in Muztagh Ata of the westernmost Tibetan Plateau. FEMS Microbiol Ecol 93: fix085. https://doi.org/10.1093/femsec/fix085
Lew S, Glińska-Lewczuk K, Ziembińska-Buczyńska A (2018) Prokaryotic community composition affected by seasonal changes in physicochemical properties of water in peat bog lakes. Water 10:485. https://doi.org/10.3390/w10040485
Longnecker K, Sherr BF, Sherr EB (2005) Activity and phylogenetic diversity of bacterial cells with high and low nucleic acid content and electron transport system activity in an upwelling ecosystem. Appl Environ Microbiol 71:7737–7749. https://doi.org/10.1128/AEM.71.12.7737-7749.2005
Read DS, Gweon HS, Bowes MJ, Newbold LK, Field D, Bailey MJ, Griffiths RI (2015) Catchment-scale biogeography of riverine bacterioplankton. ISME J 9:516–526. https://doi.org/10.1038/ismej.2014.166
Padan E, Bibi E, Ito M, Krulwich TA (2005) Alkaline pH homeostasis in bacteria: new insights. Biochim Biophys Acta 1717:67–88. https://doi.org/10.1016/j.bbamem.2005.09.010
Padan E, Zilberstein D, Rottenberg H (1976) The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem 63:533–541. https://doi.org/10.1111/j.1432-1033.1976.tb10257.x
Slonczewski JL, Rosen BP, Alger JR, Macnab RM (1981) pH homeostasis in Escherichia coli: measurement by 31P nuclear magnetic resonance of methylphosphonate and phosphate. PNAS 78:6271–6275
Booth IR (1985) Regulation of cytoplasmic pH in bacteria. Microbiol Rev 49:359–378
Baatout S, Leys N, Hendrickx L, Dams A, Mergeay M (2007) Physiological changes induced in bacteria following pH stress as a model for space research. Acta Astronaut 60:451–459. https://doi.org/10.1016/j.actaastro.2006.09.012
Albert LS, Brown DG (2015) Variation in bacterial ATP concentration during rapid changes in extracellular pH and implications for the activity of attached bacteria. Colloid Surface B 132: 111. https://doi.org/10.1016/j.colsurfb.2015.05.020
Grinius L, Slusnyte R, Griniuviene B (1975) ATP synthesis driven by protonmotive force imposed across Escherichia coli cell membranes. FEBS Lett 57:290–293. https://doi.org/10.1016/0014-5793(75)80319-X
Singh AP, Bragg PD (1979) ATP synthesis driven by a pH gradient imposed across the cell membranes of lipoic acid and unsaturated fatty acid auxotrophs of Escherichia coli. FEBS Lett 98:21–24. https://doi.org/10.1016/0014-5793(79)80142-8
Nelson WC, Stegen JC (2015) The reduced genomes of Parcubacteria (OD1) contain signatures of a symbiotic lifestyle. Front Microbiol 6:713. https://doi.org/10.3389/fmicb.2015.00713
Mclean JS, Lombardo MJ, Badger JH, Edlund A, Novotny M, Yee-Greenbaum J, Vyahhi N, Hall AP, Yang Y, Dupont CL (2013) Candidate phylum TM6 genome recovered from a hospital sink biofilm provides genomic insights into this uncultivated phylum. PNAS 110:2390–2399
Ward L, Taylor MW, Power JF, Scott BJ, Mcdonald IR, Stott MB (2017) Microbial community dynamics in Inferno Crater Lake, a thermally fluctuating geothermal spring. ISME J 11:1158–1167. https://doi.org/10.1038/ismej.2016.193
Shade A, Chiu CY, Mcmahon KD (2010) Differential bacterial dynamics promote emergent community robustness to lake mixing: an epilimnion to hypolimnion transplant experiment. Environ Microbiol 12:455–466. https://doi.org/10.1111/j.1462-2920.2009.02087.x
Yannarell AC, Triplett EW (2005) Geographic and environmental sources of variation in lake bacterial community composition. Appl Environ Microbiol 71:227–239. https://doi.org/10.1128/AEM.71.1.227-239.2005
Lindstrom ES, Kamst-Van Agterveld MP, Zwart G (2005) Distribution of typical freshwater bacterial groups is associated with pH, temperature, and lake water retention time. Appl Environ Microbiol 71:8201–8206. https://doi.org/10.1128/aem.71.12.8201-8206.2005
Wang H, Ji G, Bai X (2015) Enhanced long-term ammonium removal and its ranked contribution of microbial genes associated with nitrogen cycling in a lab-scale multimedia biofilter. Bioresour Technol 196:57–64. https://doi.org/10.1016/j.biortech.2015.07.055
Wang L, Li T (2011) Anaerobic ammonium oxidation in constructed wetlands with bio-contact oxidation as pretreatment. Ecol Eng 37:1225–1230. https://doi.org/10.1016/j.ecoleng.2011.03.008
Zhang L, Narita Y, Gao L, Ali M, Oshiki M, Ishii S, Okabe S (2017) Microbial competition among anammox bacteria in nitrite-limited bioreactors. Water Res 125:249–258. https://doi.org/10.1016/j.watres.2017.08.052
van Teeseling MC, Mesman RJ, Kuru E, Espaillat A, Cava F, Brun YV, Vannieuwenhze MS, Kartal B, van Niftrik L (2015) Anammox Planctomycetes have a peptidoglycan cell wall. Nat Commun 6:6878. https://doi.org/10.1038/ncomms7878
Godde M, Conrad R (1999) Immediate and adaptational temperature effects on nitric oxide production and nitrous oxide release from nitrification ad denitrification in two soils. Biol Fertil Soils 30:33–40. https://doi.org/10.1007/s003740050584
Holtan-Hartwig L, Dörsch P, Bakken LR (2002) Low temperature control of soil denitrifying communities: kinetics of N2O production and reduction. Soil Biol Biochem 34:1797–1806. https://doi.org/10.1016/S0038-0717(02)00169-4
Zhang L, Zeng G, Zhang J, Chen Y, Yu M, Lu L, Li H, Zhu Y, Yuan Y, Huang A (2015) Response of denitrifying genes coding for nitrite (nirK or nirS) and nitrous oxide (nosZ) reductases to different physico-chemical parameters during agricultural waste composting. Appl Microbiol Biotechnol 99:4059–4070. https://doi.org/10.1007/s00253-014-6293-3
Deiglmayr K, Philippot L, Hartwig UA, Kandeler E (2004) Structure and activity of the nitrate-reducing community in the rhizosphere of Lolium perenne and Trifolium repens under long-term elevated atmospheric pCO2. FEMS Microbiol Ecol 49:445–454. https://doi.org/10.1016/j.femsec.2004.04.017
Funding
This study was funded by the National Natural Science Foundation of China (No.31670498 and 31322012).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 357 kb)
Rights and permissions
About this article
Cite this article
Song, Y., Mao, G., Gao, G. et al. Structural and Functional Changes of Groundwater Bacterial Community During Temperature and pH Disturbances. Microb Ecol 78, 428–445 (2019). https://doi.org/10.1007/s00248-019-01333-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-019-01333-7