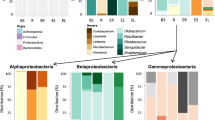
Abstract
The interaction of plants and root-associated bacteria encourage the removal of soil contaminants. Engineers and scientists have looked at this phenomenon as a possible means of soil treatment (rhizodegradation). In this study, root-associated bacteria were isolated and selected for growth on a model soil contaminant: polycyclic aromatic hydrocarbons. Isolates were compared genetically to see how plant–bacteria interactions change with soil contamination levels. Characterization of root-associated bacteria was performed using REP-PCR genetic fingerprinting and 16s rRNA gene alignments for identification. Genomic fingerprinting indicated that the composition of PAH-metabolizing bacteria (“guild”) was similar among plant species at each treatment level. However, guild composition changed with contamination level and differed from that of bulk soils, suggesting a common rhizosphere effect among plant species related to PAH contamination. PAH-metabolizing bacteria were identified through 16s rRNA gene alignment as members of the α-, β-, and γ-proteobacteria, Actinobacteria, and Bacilli classes. Burkholderia and Pseudomonas spp. were the only genera of bacteria isolated from all plant types in uncontaminated controls. Bacterial species found at the highest treatment included Achromobacter xylosoxidans, Rhodococcus spp., members of the Microbacteriae, Stenotrophomonas rhizophilia, as well as other members of the alpha-proteobacteria. Given their ability to grow on PAHs and inhabit highly contaminated rhizospheres, these bacteria appear good candidates for the promotion of rhizodegradation.





Similar content being viewed by others
References
Glick BR (2003) Phytoremediation, synergistic use of plants and bacteria to clean up the environment. Biotechnol Adv 21:383–393
Miya RK, Firestone MK (2001) Enhanced phenanthrene biodegradation in soil by slender oat root exudates and root debris. J Environ Qual 30(6):1911–1918
Kamath R, Schnoor J, Alvarez P (2004) Effect of root-derived substrates on the expression of nah-lux genes in Pseudomonas fluorescens hk44: implications for PAH biodegradation in the rhizosphere. Environ Sci Technol 38(1740):1740–1745
Knueffel DA (1993) The behavior and tracking of bacteria in the rhizosphere. Annu Rev Phytopathol 31:441–472
Dandurand LM and G.R. Knudsen (2001) Sampling Microbes from the Rhizosphere and Phyllosphere. In: Hurst CJ, et al. (eds) Manual of Environmental Microbiology. American Society for Microbiology
Duineveld BM, Kowalchuk GA, Keijzer A, van Elsas JD, van Veen JA (2001) Analysis of bacterial communities in the rhizosphere of chrysanthemum via denaturing gradient gel electrophoresis of PCR-amplified 16S rRNA as well as DNA fragments coding for 16S rRNA. Appl Environ Microbiol 67(1):172–178
Whipps JM (2001) Microbial interactions and biocontrol in the rhizosphere. J Exp Bot 52(suppl_1):487–511
Whipps JM (1990) Carbon Economy. In: Lynch JM (ed) The Rhizosphere. John Wiley and Sons, Chichester
Sims RC, Overcash MR (1983) Fate of polynuclear aromatic compounds (PNAs) in soil-plant systems. Residue Reviews 88:1–68
Shuttleworth KL, Cerniglia CE (1995) Environmental aspects of PAH biodegradation. Appl Biochem Biotechnol 54(1–3):291–302
Lepsis J, Blanke MM Environmental Effects, Phytotoxicity and Breakdown of Tar Oil From Impregnated Tree Stakes, in VIII International Symposium on Canopy, Rootstocks, and Environmental Physiology in Orchard Systems, ISHS Acta Horticulturae
Aitken MD, Stringfellow WT, Nagel RD, Kazunga C, Chen SH (1998) Characteristics of phenanthrene-degrading bacteria isolated from soils contaminated with polycyclic aromatic hydrocarbons. Can J Microbiol 44(8):743–752
Siciliano SD et al (2001) Selection of specific endophytic bacterial genotypes by plants in response to soil contamination. Appl Environ Microbiol 67(6):2469–2475
Daane LL et al (2001) Isolation and characterization of polycyclic aromatic hydrocarbon-degrading bacteria associated with the rhizosphere of salt marsh plants. Appl Environ Microbiol 67(6):2683–2691
Bastiaens L et al (2000) Isolation of adherent polycyclic aromatic hydrocarbon (PAH)-degrading bacteria using PAH-sorbing carriers. Appl Environ Microbiol 66(5):1834–1843
Parrish ZD, White JC, Isleyen M, Gent MPN, Iannucci-Berger W, Eitzer BD, Kelsey JW, Mattina MI (2006) Accumulation of weathered polycyclic aromatic hydrocarbons (PAHs) by plant and earthworm species. Chemosphere 64(4):609–618
Corgie SC, Beguiristain T, Leyval C (2004) Spatial distribution of bacterial communities and phenanthrene degradation in the rhizosphere of Lolium perenne L. Appl Environ Microbiol 70(6):3552–3557
Juhasz AL, Naidu R (2000) Enrichment and isolation of non-specific aromatic degraders from unique uncontaminated (plant and faecal material) sources and contaminated soils. J Appl Microbiol 89(4):642–650
Ramos C, Molbak L, Molin S (2000) Bacterial activity in the rhizosphere analyzed at the single-cell level by monitoring ribosome contents and synthesis rates. Appl Environ Microbiol 66(2):801–809
Bringhurst RM, Cardon ZG, Gage DJ (2001) Galactosides in the rhizosphere: utilization by Sinorhizobium meliloti and development of a biosensor. Proc Natl Acad Sci U S A 98:4540–4545
Niemi MR et al (2001) Extraction and purification of DNA in rhizosphere soil samples for PCR-DGGE analysis of bacterial consortia. J Microbiol Methods 45(3):155–165
Nunan N et al (2005) Links between plant and rhizoplane bacterial communities in grassland soils, characterized using molecular techniques. Appl Environ Microbiol 71(11):6784–6792
Pritchina O, Ely C, Smets B (2011) Effects of PAH-contaminated soil on rhizosphere microbial communities. Water Air Soil Pollut:1–9
Baudoin E, Benizri E, Guckert A (2002) Impact of growth stage on the bacterial community structure along maize roots, as determined by metabolic and genetic fingerprinting. Appl Soil Ecol 19(2):135–145
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5(3):218–223
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12(4):564–582
Pillai BVS, Swarup S (2002) Elucidation of the flavonoid catabolism pathway in Pseudomonas putida PML2 by comparative metabolic profiling. Appl Environ Microbiol 68(1):143–151
Adams N et al (2000) Introduction to phytoremediation. Environmental Protection Agency, Washington, D.C.
Ely CS, Smets BF (2017) Bacteria from wheat and cucurbit plant roots metabolize PAHs and aromatic root exudates: implications for rhizodegradation. Int J Phytoremediat 19(10):877–883
Taghavi S et al (2005) Horizontal gene transfer to endogenous endophytic bacteria from poplar improves phytoremediation of toluene. Appl Environ Microbiol 71(12):8500–8505
Ramette A, LiPuma JJ, Tiedje JM (2005) Species abundance and diversity of Burkholderia cepacia complex in the environment. Appl Environ Microbiol 71(3):1193–1201
Salles JF, van Veen JA, van Elsas JD (2004) Multivariate analyses of Burkholderia species in soil: effect of crop and land use history. Appl Environ Microbiol 70(7):4012–4020
Rosenblueth M, Martinez-Romero E (2006) Bacterial endophytes and their interactions with hosts. Mol Plant-Microbe Interact 19(8):827–837
Compant S et al (2005) Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl Environ Microbiol 71(4):1685–1693
Kahng, et al. (2002) PAH utilization by Pseudomonas rhodesiae KK1 isolated from a former manufactured-gas plant site. Appl Microbiol Biotechnol 60(4):475–480
Deziel E et al (1996) Biosurfactant production by a soil Pseudomonas strain growing on polycyclic aromatic hydrocarbons. Appl Environ Microbiol 62(6):1908–1912
MacNaughton SJ et al (1999) Microbial population changes during bioremediation of an experimental oil spill. Appl Environ Microbiol 65(8):3566–3574
Stringfellow WT, Aitken MD (1994) Comparative physiology of phenanthrene degradation by 2 dissimilar pseudomonads isolated from a creosote-contaminated soil. Can J Microbiol 40(6):432–438
Stringfellow W, Aitken M (1995) Competitive metabolism of naphthalene, methylnaphthalenes, and fluorene by phenanthrene-degrading pseudomonads. Appl Environ Microbiol 61(1):357–2392
Kazunga C, Aitken MD (2000) Products from the incomplete metabolism of pyrene by polycyclic aromatic hydrocarbon-degrading bacteria. Appl Environ Microbiol 66(5):1917–1922
Eriksson M et al (2003) Degradation of polycyclic aromatic hydrocarbons at low temperature under aerobic and nitrate-reducing conditions in enrichment cultures from northern soils. Appl Environ Microbiol 69(1):275–284
Moody JD et al (2001) Degradation of phenanthrene and anthracene by cell suspensions of Mycobacterium sp. strain PYR-1. Appl Environ Microbiol 67(4):1476–1483
Brezna B, Khan A, Cerniglia CE (2003) Molecular characterization of dioxygenases from polycyclic aromatic hydrocarbon-degrading Mycobacterium spp. FEMS Microbiol Ecol 223:177–183
Larkin MJ et al (1999) Purification and characterization of a novel naphthalene dioxygenase from Rhodococcus sp. strain NCIMB12038. J Bacteriol 181(19):6200–6204
Demaneche S et al (2004) Identification and functional analysis of two aromatic-ring-hydroxylating dioxygenases from a Sphingomonas strain that degrades various polycyclic aromatic hydrocarbons. Appl Environ Microbiol 70(11):6714–6725
Masson-Boivin M et al (2009) Establishing nitrogen-fixing symbioses with legumes: how many rhizobium recipes? Trends Microbiol 17(10):458–466
Funding
This research was performed by a funding grant from the Environmental Protection Agency Star Program, R829405.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ely, C.S., Smets, B.F. Guild Composition of Root-Associated Bacteria Changes with Increased Soil Contamination. Microb Ecol 78, 416–427 (2019). https://doi.org/10.1007/s00248-019-01326-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-019-01326-6