Abstract
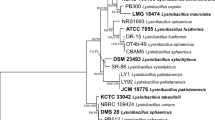
Endophytic microorganisms which are ubiquitously present in plants may colonize intracellularly or intercellularly without causing any diseases. By living within the unique chemical environment of a host plant, they produce a vast array of compounds with a wide range of biological activities. Because of this, natural products of endophytic origin have been exploited for antimicrobial, antiviral, anticancer, and antioxidant properties. Also, they can be considered to function as an efficient microbial barrier to protect plants from various pathogens. In the present study, endophytic bacterium BmB 9 with antifungal and antibacterial activity isolated from the stem tissue of Bacopa monnieri was studied for the molecular and chemical basis of its activity. PCR-based genome mining for various biosynthetic gene clusters proved the presence of surfactin, iturin, and type I polyketide synthase (PKS) genes in the isolate. The LC–MS/MS based analysis of the extract further confirmed the production of surfactin derivatives (M + H+—1008.6602, 1022.6755), iturin (M + H+—1043.5697), and fengycin (M + H+—1491.8195, 1477.8055) by the selected bacterial isolate. The 16S rDNA sequence similarity based analysis identified the isolate BmB 9 as Bacillus sp. with 100 % identity to Bacillus sp. LCF1 (KP257289).










Similar content being viewed by others
References
Jasim B, John Jimtha C, Jyothis M et al (2013) Plant growth promoting potential of endophytic bacteria isolated from Piper nigrum. Plant Growth Regul 71:1–11. doi:10.1007/s10725-013-9802-y
Moyne A-L, Cleveland TE, Tuzun S (2004) Molecular characterization and analysis of the operon encoding the antifungal lipopeptide bacillomycin D. FEMS Microbiol Lett 234:43–49. doi:10.1016/j.femsle.2004.03.011
Roongsawang N, Washio K, Morikawa M (2011) Diversity of nonribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants. Int J Mol Sci 12:141–172. doi:10.3390/ijms12010141
Deleu M, Razafindralambo H, Popineau Y et al (1999) Interfacial and emulsifying properties of lipopeptides from Bacillus subtilis. Colloids Surfaces A Physicochem Eng Asp 152:3–10. doi:10.1016/S0927-7757(98)00627-X
Peypoux F, Bonmatin J, Wallach J (1999) Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol 51:553–563
Straus SK, Hancock REW (2006) Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: comparison with cationic antimicrobial peptides and lipopeptides. Biochim Biophys Acta - Biomembr 1758:1215–1223. doi:10.1016/j.bbamem.2006.02.009
Jasim B, Anisha C, Rohini S et al (2013) Phenazine carboxylic acid production and rhizome protective effect of endophytic Pseudomonas aeruginosa isolated from Zingiber officinale. World J Microbiol Biotechnol. doi:10.1007/s11274-013-1582-z
Jasim B, Jimtha John C, Shimil V et al (2014) Studies on the factors modulating indole-3-acetic acid production in endophytic bacterial isolates from Piper nigrum and molecular analysis of ipdc gene. J Appl Microbiol 117:786–799. doi:10.1111/jam.12569
Thomas R, Nair A, KR S et al (2014) Antibacterial activity and synergistic effect of biosynthesized AgNPs with antibiotics against multidrug-resistant biofilm-forming coagulase-negative staphylococci isolated from clinical samples. Appl Biochem Biotechnol 173:449–460. doi:10.1007/s12010-014-0852-z
Jasim B, Anish M, Shimil V et al (2015) Studies on plant growth promoting properties of fruit-associated bacteria from Elettaria cardamomum and molecular analysis of ACC deaminase gene. Appl Biochem Biotechnol 177:175–189. doi:10.1007/s12010-015-1736-6
Long HH, Schmidt DD, Baldwin IT (2008) Native bacterial endophytes promote host growth in a species-specific manner; phytohormone manipulations do not result in common growth responses. PLoS One 3:e2702. doi:10.1371/journal.pone.0002702
Goryluk A, Rekosz-Burlaga H, Błaszczyk M (2009) Isolation and characterization of bacterial endophytes of Chelidonium majus L. Polish J Microbiol 58:355–361
Thomashow LS, Weller DM (1988) Role of a phenazine antibiotic from Pseudomonas fluorescens in biological control of Gaeumannomyces graminis var. tritici. J Bacteriol 170:3499–3508
Kempf H, Wolf G (1989) Erwinia herbicola as a bio-control agent of Fusarium culmorum and Puccinia recondita f. sp. Tritici on wheat. Phytopathology 79:990–994
Steller S, Vollenbroich D, Leenders F et al (1999) Structural and functional organization of the fengycin synthetase multienzyme system from Bacillus subtilis b213 and A1/3. Chem Biol 6:31–41. doi:10.1016/S1074-5521(99)80018-0
Stein T (2005) Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol 56:845–857. doi:10.1111/j.1365-2958.2005.04587.x
Cho S-J, Lee SK, Cha BJ et al (2003) Detection and characterization of the Gloeosporium gloeosporioides growth inhibitory compound iturin A from Bacillus subtilis strain KS03. FEMS Microbiol Lett 223:47–51
Wei Y-H, Wang L-C, Chen W-C, Chen S-Y (2010) Production and characterization of fengycin by indigenous Bacillus subtilis F29-3 originating from a potato farm. Int J Mol Sci 11:4526–4538. doi:10.3390/ijms11114526
Tang J-S, Zhao F, Gao H et al (2010) Characterization and online detection of surfactin isomers based on HPLC-MS(n) analyses and their inhibitory effects on the overproduction of nitric oxide and the release of TNF-α and IL-6 in LPS-induced macrophages. Mar Drugs 8:2605–2618. doi:10.3390/md8102605
Oehrle N, Karr D, Kremer R, Emerich D (2000) Enhanced attachment of Bradyrhizobium japonicum to soybean through reduced root colonization of internally seedborne microorganisms. Can J Microbiol 46:600–606
Rajendran G, Sing F, Desai AJ, Archana G (2008) Enhanced growth and nodulation of pigeon pea by co-inoculation of Bacillus strains with Rhizobium spp. Bioresour Technol 99:4544–4550. doi:10.1016/j.biortech.2007.06.057
Selvakumar G, Kundu S, Gupta A et al (2008) Isolation and characterization of nonrhizobial plant growth promoting bacteria from nodules of kudzu (Pueraria thunbergiana) and their effect on wheat seedling growth. Curr Microbiol 56:134–139. doi:10.1007/s00284-007-9062-z
Jasim B, Geethu PR, Mathew J, Radhakrishnan EK (2015) Effect of endophytic Bacillus sp. from selected medicinal plants on growth promotion and diosgenin production in Trigonella foenum-graecum. Plant Cell, Tissue Organ Cult 122:565–572. doi:10.1007/s11240-015-0788-1
Korzybski T, Kowszyk-Gifinder Z, Kurytowicz W (1978) Antibiotics: origin, nature, and properties. American Society for Microbiology. American Society for Microbiology, Washington, D.C.
Naruse N, Tenmyo O, Kobaru S (1990) Pumilacidin, a complex of new antiviral antibiotics: production, isolation, chemical properties, structure and biological activity. J Antibiot (Tokyo) 43:267–280
Munimbazi B (1998) Isolation and partial characterization of antifungal metabolites of Bacillus pumilus. J Appl Microbiol 84:959–968. doi:10.1046/j.1365-2672.1998.00431.x
Pabel CT, Vater J, Wilde C et al (2003) Antimicrobial activities and matrix-assisted laser desorption/ionization mass spectrometry of Bacillus isolates from the marine sponge Aplysina aerophoba. Mar Biotechnol 5:424–434. doi:10.1007/s10126-002-0088-8
Tamehiro N, Okamoto-Hosoya Y, Okamoto S et al (2002) Bacilysocin, a novel phospholipid antibiotic produced by Bacillus subtilis 168. Antimicrob Agents Chemother 46:315–320. doi:10.1128/AAC.46.2.315-320.2002
Zheng G, Slavik MF (1999) Isolation, partial purification and characterization of a bacteriocin produced by a newly isolated Bacillus subtilis strain. Lett Appl Microbiol 28:363–367. doi:10.1046/j.1365-2672.1999.00545.x
Arrebola E, Jacobs R, Korsten L (2010) Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J Appl Microbiol 108:386–395. doi:10.1111/j.1365-2672.2009.04438.x
Hsieh F-C, Lin T-C, Meng M, Kao S-S (2008) Comparing methods for identifying bacillus strains capable of producing the antifungal lipopeptide iturin A. Curr Microbiol 56:1–5. doi:10.1007/s00284-007-9003-x
Yu GY, Sinclair JB, Hartman GL, Bertagnolli BL (2002) Production of iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem 34:955–963. doi:10.1016/S0038-0717(02)00027-5
Koumoutsi A, Chen X-H, Henne A et al (2004) Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J Bacteriol 186:1084–1096. doi:10.1128/JB.186.4.1084-1096.2004
Romero D, de Vicente A, Rakotoaly RH et al (2007) The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol Plant Microbe Interact 20:430–40. doi:10.1094/MPMI-20-4-0430
Mounia Y-A, Noreddine KC, Laid D et al (2014) Antifungal activity and bioactive compounds produced by Bacillus mojavensis and Bacillus subtilis. African J Microbiol Res 8:476–484. doi:10.5897/AJMR2013.6327
Snook ME, Mitchell T, Hinton DM, Bacon CW (2009) Isolation and characterization of Leu7-surfactin from the endophytic bacterium Bacillus mojavensis RRC 101, a biocontrol agent for Fusarium verticillioides. J Agric Food Chem 57:4287–4292. doi:10.1021/jf900164h
Bacon CW, Hinton DM, Mitchell TR et al (2012) Characterization of endophytic strains of Bacillus mojavensis and their production of surfactin isomers. Biol Control 62:1–9. doi:10.1016/j.biocontrol.2012.03.006
Marrone P (2002) An effective biofungicide with novel modes of action. Pestic outlook 13:193–194
Acknowledgment
This study was supported by Department of Biotechnology (DBT), Government of India under DBT-RGYI and DBT-MSUB support scheme (BT/PR4800/INF/22/152/2012 Dtd 23.03.2012) and Kerala State Council Science Technology and Environment (KSCSTE) under KSCSTE-SARD Programme and KSCSTE-SRS Major Project. The authors also acknowledge Prof. C. T. Aravindakumar, Hon. Director and Mr. Dineep D., Scientific Assistant of the Inter-University Instrumentation Centre, Mahatma Gandhi University, Kottayam for the help and support in the LC-MS/MS analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Standards
We have not used any animal models for the experiments, thus, an ethical committee clearance was not required.
Conflict of Interest
The authors declare no conflict of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 8889 kb)
Rights and permissions
About this article
Cite this article
Jasim, B., Sreelakshmi, K.S., Mathew, J. et al. Surfactin, Iturin, and Fengycin Biosynthesis by Endophytic Bacillus sp. from Bacopa monnieri . Microb Ecol 72, 106–119 (2016). https://doi.org/10.1007/s00248-016-0753-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0753-5