Abstract
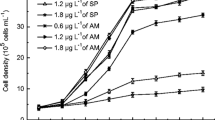
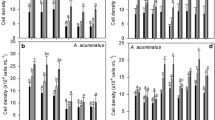
Coexisting antibiotic contaminants have potential to regulate cyanobacterial bloom, and the regulation is likely affected by nitrogen supply. A typical cyanobaterium Microcystis aeruginosa was cultured with 0.05–50 mg L−1 of nitrogen and exposed to 100–600 ng L−1 of amoxicillin for 7 days. Algal growth was not significantly (p > 0.05) affected by amoxicillin at the lowest nitrogen level of 0.05 mg L−1, stimulated by 600 ng L−1 of amoxicillin at a moderate nitrogen level of 0.5 mg L−1 and enhanced by 100–600 ng L−1 of amoxicillin at higher nitrogen levels of 5–50 mg L−1. Amoxicillin affected chlorophyll-a, psbA gene, and rbcL gene in a similar manner as algal growth, suggesting a regulation of algal growth via the photosynthesis system. At each nitrogen level, synthesis of protein and polysaccharides as well as production and release of microcystins (MCs) increased in response to environmental stress caused by amoxicillin. Expression of ntcA and mcyB showed a positive correlation with the total content of MCs under exposure to amoxicillin at nitrogen levels of 0.05–50 mg L−1. Nitrogen and amoxicillin significantly (p < 0.05) interact with each other on the regulation of algal growth, synthesis of chlorophyll-a, production and release of MCs, and expression of ntcA and mcyB. The nitrogen-dependent stimulation effect of coexisting amoxicillin contaminant on M. aeruginosa bloom should be fully considered during the combined pollution control of M. aeruginosa and amoxicillin.




Similar content being viewed by others
References
Paerl HW, Huisman J (2009) Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ Microbiol Rep 1:27–37
Graham JL, Loetin KA, Meyer MT, Ziegler AA (2010) Cyanotoxin mixtures and taste-and-odor compounds in cyanobacteria blooms from the Midwestern United States. Environ Sci Technol 44:7361–7368
Falconer IR, Humpage AR (2005) Health risk assessment of cyanobacterial (blue-green algal) toxins in drinking water. Int J Environ Res Public Health 2:43–50
Davis TW, Berry DL, Boyer GL, Gobler CJ (2009) The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 8:715–725
Jiang Y, Ji B, Wong RNS, Wong MH (2008) Statistical study on the effects of environmental factors on the growth and microcystins production of bloom-forming cyanobacterium—Microcystis aeruginosa. Harmful Algae 7:127–136
Downing TG, Sember CS, Gehringer MM, Leukes W (2005) Medium N:P ratios and specific growth rate comodulate microcystin and protein content in Microcystis aeruginosa PCC7806 and M. aeruginosa UV027. Microb Ecol 49:468–473
Shao JH, Wu ZX, Yu GL, Peng X, Li RH (2009) Allelopathic mechanism of pyrogallol to Microcystis aeruginosa PCC7806 (Cyanobacteria): from views of gene expression and antioxidant system. Chemosphere 75:924–928
Babica P, Bláha L, Maršálek B (2006) Exploring the natural role of microcystins—a review of effects on photoautotrophic organisms. J Phycol 42:9–20
Ma J (2005) Differential sensitivity of three cyanobacterial and five green algal species to organotins and pyrethroids pesticides. Sci Total Environ 341:109–117
Hu X, Liu YG, Zeng GM, Hu XJ, Wang YQ, Zeng XX (2014) Effects of limonene stress on the growth of and microcystin release by the freshwater cyanobacterium Microcystis aeruginosa FACHB-905. Ecotox Environ Safe 105:121–127
Wang SZ, Zhang DY, Pan XL (2012) Effects of arsenic on growth and photosystem II (PSII) activity of Microcystis aeruginosa. Ecotox Environ Safe 84:104–111
Zhou WB, Juneau P, Qiu BS (2006) Growth and photosynthetic responses of the bloom-forming cyanobacterium Microcystis aeruginosa to elevated levels of cadmium. Chemosphere 65:1738–1746
Qian HF, Pan XJ, Chen J, Zhou DM, Chen ZG, Zhang L, Fu ZW (2012) Analyses of gene expression and physiological changes in Microcystis aeruginosa reveal the phytotoxicities of three environmental pollutants. Ecotoxicology 21:847–859
Zhu XZ, Kong HL, Gao YZ, Wu MF, Kong FX (2012) Low concentrations of polycyclic aromatic hydrocarbons promote the growth of Microcystis aeruginosa. J Hazard Mater 237:371–375
Liu Y, Gao BY, Yue QY, Guan YT, Wang Y, Huang LH (2012) Influences of two antibiotic contaminants on the production, release and toxicity of microcystins. Ecotox Environ Safe 77:79–87
Rijstenbil JW, Dehairs F, Ehrlich R, Wijnholds JA (1998) Effect of the nitrogen status on copper accumulation and pools of metal-binding peptides in the planktonic diatom Thalassiosira pseudonana. Aquat Toxicol 42:187–209
Kong QX, Zhu LZ, Shen XY (2010) The toxicity of naphthalene to marine Chlorella vulgaris under different nutrient conditions. J Hazard Mater 178:282–286
Chalifour A, Juneau P (2011) Temperature-dependent sensitivity of growth and photosynthesis of Scenedesmus obliquus, Navicula pelliculosa and two strains of Microcystis aeruginosa to the herbicide atrazine. Aquat Toxicol 103:9–17
Braford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye biding. Anal Biochem 72:248–254
Yang Z, Geng L, Wang W, Zhang J (2012) Combined effects of temperature, light intensity, and nitrogen concentration on the growth and polysaccharide content of Microcystis aeruginosa in batch culture. Biochem Syst Ecol 41:130–135
Tao Y, Mao X, Hu J, Mok HOL, Wang L, Au DWT, Zhu J, Zhang XX (2013) Mechanisms of photosynthetic inactivation on growth suppression of Microcystis aeruginosa under UV-C stress. Chemosphere 93:637–644
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Horii T, Mase K, Suzuki Y, Kimura T, Ohta M, Maekawa M, Kanno T, Kobayashi M (2002) Antibacterial activities of β-lactamase inhibitors associated with morphological changes of cell wall in Helicobacter pylori. Helicobacter 7:39–45
Stevens DL, Yan S, Bryant AE (1993) Penicillin-binding protein expression at different growth stages determines penicillin efficacy in vitro and in vivo: an explanation for the inoculum effect. J Infect Dis 167:1401–1405
Marbouty M, Mazouni K, Saguez C, Cassier-Chauvat C, Chauvat F (2009) Characterization of the Synechocystis strain PCC 6803 penicillin-binding proteins and cytokinetic proteins FtsQ and FtsW and their network of interactions with ZipN. J Bacteriol 191:5123–5133
de Morais P, Stoichev T, Basto MCP, Ramos V, Vasconcelos VM, Vasconcelos MTSD (2014) Cyanobacterium Microcystis aeruginosa response to pentachlorophenol and comparison with that of the microalga Chlorella vulgaris. Water Res 52:63–72
Qiu HM, Geng JJ, Ren HQ, Xia XM, Wang XR, Yu Y (2013) Physiological and biochemical responses of Microcystis aeruginosa to glyphosate and its Roundup® formulation. J Hazard Mater 248:172–176
Wang JX, Xie P, Guo N (2007) Effects of nonylphenol on the growth and microcystin production of Microcystis strains. Environ Res 103:70–78
Stoichev T, Baptista MS, Basto MCP, Vasconcelos VM, Vasconcelos MTSD (2011) Effects of minocycline and its degradation products on the growth of Microcystis aeruginosa. Ecotox Environ Safe 74:219–224
Pomati F, Netting AG, Calamari D, Neilan BA (2004) Effects of erythromycin, tetracycline and ibuprofen on the growth of Synechocystis sp. and Lemna minor. Aquat Toxicol 67:387–396
Liu Y, Guan YT, Gao BY, Yue QY (2012) Antioxidant responses and degradation of two antibiotic contaminants in Microcystis aeruginosa. Ecotox Environ Safe 86:23–30
Aro EM, Virgin I, Andersson B (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. BA-Bioenerg 1143:113–134
Pan XL, Deng CN, Zhang DY, Wang JL, Mu GJ, Chen Y (2008) Toxic effects of amoxicillin on the photosystem II of Synechocystis sp characterized by a variety of in vivo chlorophyll fluorescence tests. Aquat Toxicol 89:207–213
Feller U, Anders I, Mae T (2008) Rubiscolytics: fate of Rubisco after its enzymatic function in a cell is terminated. J Exp Bot 59:1615–1624
Kong Y, Zou P, Yang Q, Xu XY, Miao LH, Zhu L (2013) Physiological responses of Microcystis aeruginosa under the stress of antialgal actinomycetes. J Hazard Mater 262:274–280
Ye J, Wang LM, Zhang ZJ, Liu WP (2013) Enantioselective physiological effects of the herbicide diclofop on cyanobacteriurn Microcystis aeruginosa. Environ Sci Technol 47:3893–3901
Liu ZQ, Cui FY, Ma H, Fan ZQ, Zhao ZW, Hou ZL, Liu DM (2014) The transformation mechanism of nitrobenzene in the present of a species of cyanobacteria Microcystis aeruginosa. Chemosphere 95:234–240
Zhang ZS, Wang F, Wang XM, Liu XL, Hou Y, Zhang QB (2010) Extraction of the polysaccharides from five algae and their potential antioxidant activity in vitro. Carbohydr Polym 82:118–121
Luque I, Flores E, Herrero A (1994) Molecular mechanism for the operation of nitrogen control in cyanobacteria. EMBO J 13:2862–2869
Sauer J, Margit G, Forchhammer K (1999) Nitrogen starvation in Synechococcus PCC 7942: involvement of glutamine synthetase and NtcA in phycobiliprotein degradation and survival. Arch Microbiol 172:247–255
Ginn HP, Pearson LA, Neilan BA (2010) NtcA from Microcystis aeruginosa PCC 7806 is autoregulatory and binds to the microcystin promoter. Appl Environ Microbiol 76:4362–4368
Hu X, Liu YG, Zeng GM, Xu WH, Hu XJ, Zhu ZL, Zhang PY, Wang YQ (2014) Effects of d-menthol stress on the growth of and microcystin release by the freshwater cyanobacterium Microcystis aeruginosa FACHB-905. Chemosphere 113:30–35
Polyak Y, Zaytseva T, Medvedeva N (2013) Response of toxic cyanobacterium Microcystis aeruginosa to environmental pollution. Water Air Soil Pollut 224:1494–1508
Zuccato E, Castiglioni S, Bagnati R, Melis M, Fanelli R (2010) Source, occurrence and fate of antibiotics in the Italian aquatic environment. J Hazard Mater 179:1042–1048
Acknowledgments
This work was supported by National Natural Science Foundation of China (51209125) and partly by Doctoral Foundation of Ministry of Education of China (20110131120014) and Promotive Research Foundation of Shandong Province (2013BSE27073).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Chen, X., Zhang, J. et al. Hormesis Effects of Amoxicillin on Growth and Cellular Biosynthesis of Microcystis aeruginosa at Different Nitrogen Levels. Microb Ecol 69, 608–617 (2015). https://doi.org/10.1007/s00248-014-0528-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-014-0528-9