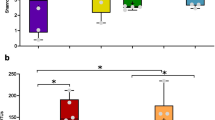
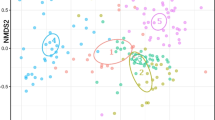
Abstract
The Brazilian endemic scleractinian corals, genus Mussismilia, are among the main reef builders of the South Atlantic and are threatened by accelerating rates of disease. To better understand how holobiont microbial populations interact with corals during health and disease and to evaluate whether selective pressures in the holobiont or neutral assembly shape microbial composition, we have examined the microbiota structure of Mussismilia corals according to coral lineage, environment, and disease/health status. Microbiota of three Mussismilia species (Mussismilia harttii, Mussismilia hispida, and Mussismilia braziliensis) was compared using 16S rRNA pyrosequencing and clone library analysis of coral fragments. Analysis of biological triplicates per Mussismilia species and reef site allowed assessment of variability among Mussismilia species and between sites for M. braziliensis. From 173,487 V6 sequences, 6,733 coral- and 1,052 water-associated operational taxonomic units (OTUs) were observed. M. braziliensis microbiota was more similar across reefs than to other Mussismilia species microbiota from the same reef. Highly prevalent OTUs were more significantly structured by coral lineage and were enriched in Alpha- and Gammaproteobacteria. Bacterial OTUs from healthy corals were recovered from a M. braziliensis skeleton sample at twice the frequency of recovery from water or a diseased coral suggesting the skeleton is a significant habitat for microbial populations in the holobiont. Diseased corals were enriched with pathogens and opportunists (Vibrios, Bacteroidetes, Thalassomonas, and SRB). Our study examines for the first time intra- and inter-specific variability of microbiota across the genus Mussismilia. Changes in microbiota may be useful indicators of coral health and thus be a valuable tool for coral reef management and conservation.




Similar content being viewed by others
References
Wiebe WJ, Johannes RE, Webb KL (1975) Nitrogen fixation in a coral reef community. Science 188:257–259. doi:10.1126/science.188.4185.257
Lesser MP, Mazel CH, Gorbunov MY, Falkowski PG (2004) Discovery of symbiotic nitrogen-fixing cyanobacteria in corals. Science 305:997–1000. doi:10.1126/science.1099128305/5686/997
Raina JB, Tapiolas D, Willis BL, Bourne DG (2009) Coral-associated bacteria and their role in the biogeochemical cycling of sulfur. Appl Environ Microbiol 75:3492–3501. doi:10.1128/AEM.02567-08
Ritchie KB (2006) Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar Ecol Prog Ser 322:1–14
Nissimov J, Rosenberg E, Munn CB (2009) Antimicrobial properties of resident coral mucus bacteria of Oculina patagonica. FEMS Microbiol Lett 292:210–215. doi:10.1111/j.1574-6968.2009.01490.x
Mao-Jones J, Ritchie KB, Jones LE, Ellner SP (2010) How microbial community composition regulates coral disease development. PLoS Biol 8:e1000345. doi:10.1371/journal.pbio.1000345
Rypien KL, Ward JR, Azam F (2010) Antagonistic interactions among coral-associated bacteria. Environ Microbiol 12:28–39. doi:10.1111/J.1462-2920.2009.02027.X
Rohwer F, Seguritan V, Azam F, Knowlton N (2002) Diversity and distribution of coral-associated bacteria. Mar Ecol-Progress Ser 243:1–10
Frias-Lopez J, Zerkle AL, Bonheyo GT, Fouke BW (2002) Partitioning of bacterial communities between seawater and healthy, black band diseased, and dead coral surfaces. Appl Environ Microbiol 68:2214–2228
Frias-Lopez J, Klaus JS, Bonheyo GT, Fouke BW (2004) Bacterial community associated with black band disease in corals. Appl Environ Microbiol 70:5955–5962. doi:10.1128/AEM.70.10.5955-5962.2004
Sunagawa S, Desantis TZ, Piceno YM, Brodie EL, Desalvo MK, Voolstra CR, Weil E, Andersen GL, Medina M (2009) Bacterial diversity and White Plague Disease-associated community changes in the Caribbean coral Montastraea faveolata. ISME J 3:512–521. doi:10.1038/ismej.2008.131
Lesser MP, Bythell JC, Gates RD, Johnstone RW, Hoegh-Guldberg O (2007) Are infectious diseases really killing corals? Alternative interpretations of the experimental and ecological data. J Exp Mar Biol Ecol 346:36–44
Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I (2007) The role of microorganisms in coral health, disease and evolution. Nat Rev Microbiol 5:355–362. doi:10.1038/nrmicro1635
Bourne DG, Garren M, Work TM, Rosenberg E, Smith GW, Harvell CD (2009) Microbial disease and the coral holobiont. Trends in Microbiol 17:554–562
Mouchka ME, Hewson I, Harvell CD (2010) Coral-associated bacterial assemblages: current knowledge and the potential for climate-driven impacts. Integrative and Comparative Biology. doi:10.1093/icb/icq061, 1–13
Francini RB, Moura RL, Thompson FL, Reis RM, Kaufman L, Kikuchi RKP, Leao ZM (2008) Diseases leading to accelerated decline of reef corals in the largest South Atlantic reef complex (Abrolhos Bank, eastern Brazil). Mar Pollut Bull 56:1008–1014
Leao ZMAN, Kikuchi RKP, Testa T (2003) Corals and coral reefs of Brazil. Elsevier, Amsterdam
Nunes F, Fukami H, Vollmer SV, Norris RD, Knowlton N (2008) Re-evaluation of the systematics of the endemic corals of Brazil by molecular data. Coral Reefs 27:423–432
Leao ZMAN, Kikuchi RKP (2005) A relic coral fauna threatened by global changes and human activities, Eastern Brazil. Mar Pollut Bull 51:599–611
Reis AM, Araujo SD Jr, Moura RL, Francini-Filho RB, Pappas G Jr, Coelho AM, Kruger RH, Thompson FL (2009) Bacterial diversity associated with the Brazilian endemic reef coral Mussismilia braziliensis. J Appl Microbiol 106:1378–1387. doi:10.1111/j.1365-2672.2008.04106.x
de Castro AP, Araujo SD Jr, Reis AM, Moura RL, Francini-Filho RB, Pappas G Jr, Rodrigues TB, Thompson FL, Kruger RH (2010) Bacterial community associated with healthy and diseased reef coral Mussismilia hispida from eastern Brazil. Microb Ecol 59:658–667. doi:10.1007/s00248-010-9646-1
Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI (2007) The human microbiome project. Nature 449:804–810. doi:10.1038/nature06244
Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Dore J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J, Bork P, Ehrlich SD (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464:59–65. doi:10.1038/nature08821
Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, Rawls JF (2011) Evidence for a core gut microbiota in the zebrafish. ISME J 5:1595–1608. doi:10.1038/ismej.2011.38
Littman RA, Willis BL, Pfeffer C, Bourne DG (2009) Diversities of coral-associated bacteria differ with location, but not species, for three acroporid corals on the Great Barrier Reef. Fems Microbiol Ecol 68:152–163. doi:10.1111/J.1574-6941.2009.00666.X
Grasshoff K, Kremling K, Erhardt M (1999) Methods of seawater analysis. Wiley, New York
Andrade L, Gonzalez AM, Araujo FV, Paranhos R (2003) Flow cytometry assessment of bacterioplankton in tropical marine environments. J Microbiol Methods 19:89–94
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–147
Stackebrandt E, Liesack W (1993) Nucleic acids and classification. In: Goodfellow M, O'Donnell AG (eds) Handbook of new bacterial systematics. Academic, London, England, pp 152–189
Watanabe K, Kodama Y, Harayama S (2001) Design and evaluation of PCR primers to amplify bacterial 16S ribosomal DNA fragments used for community fingerprinting. J Microbiological Methods 44:253–262. doi:10.1016/S0167-7012(01)00220-2
Huber T, Faulkner G, Hugenholtz P (2004) Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317–2319. doi:10.1093/bioinformatics/bth226
Nawrocki EP, Kolbe DL, Eddy SR (2009) Infernal 1.0: inference of RNA alignments. Bioinformatics 25:1335–1337. doi:10.1093/bioinformatics/btp157
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Letunic I, Bork P (2007) Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23:127–128
Sogin ML, Morrison HG, Huber JA, Welch DM, Huse SM, Neal PR, Arrieta JM, Herndl GJ (2006) Microbial diversity in the deep sea and the underexplored “rare biosphere”. Proc Natl Acad Sci U S A 103:12115–12120. doi:10.1073/pnas.0605127103
Kysela DT, Palacios C, Sogin ML (2005) Serial analysis of V6 ribosomal sequence tags (SARST-V6): a method for efficient, high-throughput analysis of microbial community composition. Environ Microbiol 7:356–364. doi:10.1111/J.1462-2920.2004.00712.X
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. doi:10.1093/bioinformatics/btr381
Huse SM, Welch DM, Morrison HG, Sogin ML (2010) Ironing out the wrinkles in the rare biosphere through improved OTU clustering. Environ Microbiol 12:1889–1898. doi:10.1111/j.1462-2920.2010.02193.x
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. doi:10.1128/AEM.00062-07
Claesson MJ, O'Sullivan O, Wang Q, Nikkila J, Marchesi JR, Smidt H, de Vos WM, Ross RP, O'Toole PW (2009) Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS ONE 4:e6669. doi:10.1371/journal.pone.0006669
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi:10.1128/AEM.01541-09
Sloan WT, Lunn M, Woodcock S, Head IM, Nee S, Curtis TP (2006) Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ Microbiol 8:732–740
Friedman J (2012) PySurvey 0.1.2: Interactive analysis of survey data. URL http://yonatanfriedman.com/docs/survey/index.html. Cambridge.
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693
Clarke KR, Chapman MG, Somerfield PJ, Needham HR (2006) Dispersion-based weighting of species counts in assemblage analyses. Mar Ecol Prog Ser 320:11–27
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust Ecol 18:117–143
Clarke KR, Warwick RM (2001) Change in marine communities: an approach to statistical analysis and interpretation. PRIMER-Ltd, Plymouth, UK
Klaus JS, Janse I, Heikoop JM, Sanford RA, Fouke BW (2007) Coral microbial communities, zooxanthellae and mucus along gradients of seawater depth and coastal pollution. Environ Microbiol 9:1291–1305. doi:10.1111/J.1462-2920.2007.01249.X
Sunagawa S, Woodley CM, Medina M (2010) Threatened corals provide underexplored microbial habitats. PLoS ONE 5:1–7. doi:10.1371/journal.pone.0009554
Chao A (1984) Non-parametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Chao A, Lee SM (1992) Estimating the number of classes via sample coverage. J Am Stat Assoc 87:210–217
Chao A, Ma MC, Yang MCK (1993) Stopping rules and estimation for recapture debugging with unequal failure rates. Biometrika 80:193–201
Hubbell SP (2001) The unified neutral theory of biodiversity and biogeography. Princeton University Press, Princeton, NJ
van der Gast CJ, Ager D, Lilley AK (2008) Temporal scaling of bacterial taxa is influenced by both stochastic and deterministic ecological factors. Environ Microbiol 10:1411–1418
de Castro AP, Araujo SD, Reis AMM, Pompeu M, Hatay M, de Moura RL, Francini-Filho RB, Thompson FL, Kruger RH (2013) Bacterial communities associated with three Brazilian endemic reef corals (Mussismilia spp.) in a coastal reef of the Abrolhos shelf. Cont Shelf Res 70:135–139
Fine M, Roff G, Ainsworth TD, Hoegh-Guldberg O (2006) Phototrophic microendoliths bloom during coral “white syndrome”. Coral Reefs 25:577–581. doi:10.1007/S00338-006-0143-4
Carilli JE, Godfrey J, Norris RD, Sandin SA, Smith JE (2010) Periodic endolithic algal blooms in Montastraea faveolata corals may represent periods of low-level stress. Bull Mar Sci 86:709–718
Lukas KJ (1974) 2 species of chlorophyte genus Ostreobium from skeletons of Atlantic and Caribbean reef corals. J Phycol 10:331–335. doi:10.1111/J.0022-3646.1974.00331.X
Gutner-Hoch E, Fine M (2011) Genotypic diversity and distribution of Ostreobium quekettii within scleractinian corals. Coral Reefs 30(Gutner-Hoch E, Fine M):643–650. doi:10.1007/S00338-011-0750-6
Bourne DG, Muirhead A, Sato Y (2011) Changes in sulfate-reducing bacterial populations during the onset of black band disease. ISME J 5:559–564. doi:10.1038/ismej.2010.143
Thompson FL, Barash Y, Sawabe T, Sharon G, Swings J, Rosenberg E (2006) Thalassomonas loyana sp nov, a causative agent of the white plague-like disease of corals on the Eilat coral reef. Int J Syst and Evol Microbiol 56:365–368. doi:10.1099/Ijs.0.63800-0
Ben-Haim Y, Thompson FL, Thompson CC, Cnockaert MC, Hoste B, Swings J, Rosenberg E (2003) Vibrio coralliilyticus sp nov., a temperature-dependent pathogen of the coral Pocillopora damicornis. Int J of Syst Evol Microbiol 53:309–315
Cervino JM, Hayes RL, Polson SW, Polson SC, Goreau TJ, Martinez RJ, Smith GW (2004) Relationship of Vibrio species infection and elevated temperatures to yellow blotch/band disease in Caribbean corals. Appl Environ Microbiol 70:6855–6864. doi:10.1128/AEM.70.11.6855-6864.2004
Sussman M, Willis BL, Victor S, Bourne DG (2008) Coral pathogens identified for White Syndrome (WS) epizootics in the Indo-Pacific. PLoS ONE 3:e2393. doi:10.1371/journal.pone.0002393
Santos Ede O, Alves N Jr, Dias GM, Mazotto AM, Vermelho A, Vora GJ, Wilson B, Beltran VH, Bourne DG, Le Roux F, Thompson FL (2011) Genomic and proteomic analyses of the coral pathogen Vibrio coralliilyticus reveal a diverse virulence repertoire. ISME J 5:1471–1483. doi:10.1038/ismej.2011.19
Leibold MA, McPeek MA (2006) Coexistence of the niche and neutral perspectives in community ecology. Ecology 87:1399–1410
Ofiteru ID, Lunn M, Curtis TP, Wells GF, Criddle CS, Francis CA, Sloan WT (2010) Combined niche and neutral effects in a microbial wastewater treatment community. Proc Natl Acad Sci U S A 107:15345–15350. doi:10.1073/pnas.1000604107
Dumbrell AJ, Nelson M, Helgason T, Dytham C, Fitter AH (2010) Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J 4:337–345. doi:10.1038/ismej.2009.122
Jeraldo P, Sipos M, Chia N, Brulc JM, Dhillon AS, Konkel ME, Larson CL, Nelson KE, Qu A, Schook LB, Yang F, White BA, Goldenfeld N (2012) Quantification of the relative roles of niche and neutral processes in structuring gastrointestinal microbiomes. Proc Natl Acad Sci U S A 109:9692–9698. doi:10.1073/pnas.1206721109
Pedron T, Mulet C, Dauga C, Frangeul L, Chervaux C, Grompone G, Sansonetti PJ (2011) A crypt-specific core microbiota resides in the mouse colon. MBio 3:116–112. doi:10.1128/mBio.00116-12
Sekelja M, Berget I, Naes T, Rudi K (2011) Unveiling an abundant core microbiota in the human adult colon by a phylogroup-independent searching approach. ISME J 5:519–531. doi:10.1038/ismej.2010.129
Schmitt S, Tsai P, Bell J, Fromont J, Ilan M, Lindquist N, Perez T, Rodrigo A, Schupp PJ, Vacelet J, Webster N, Hentschel U, Taylor MW (2012) Assessing the complex sponge microbiota: core, variable and species-specific bacterial communities in marine sponges. ISME J 6:564–576. doi:10.1038/ismej.2011.116
Wong S, Waldrop T, Summerfelt S, Davidson J, Barrows F, Kenney PB, Welch T, Wiens GD, Snekvik K, Rawls JF, Good C (2013) Aquacultured rainbow trout (Oncorhynchus mykiss) possess a large core intestinal microbiota that is resistant to variation in diet and rearing density. Appl Environ Microbiol 79:4974–4984. doi:10.1128/AEM.00924-13
Dishaw LJ, Flores-Torres J, Lax S, Gemayel K, Leigh B, Melillo D, Mueller MG, Natale L, Zucchetti I, De Santis R, Pinto MR, Litman GW, Gilbert JA (2014) The gut of geographically disparate Ciona intestinalis harbors a core microbiota. Plos One 9:e93386. doi:10.1371/journal.pone.0093386
Burke C, Thomas T, Lewis M, Steinberg P, Kjelleberg S (2011) Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. ISME J 5:590–600. doi:10.1038/ismej.2010.164
Wong AC, Chaston JM, Douglas AE (2013) The inconstant gut microbiota of Drosophila species revealed by 16S rRNA gene analysis. ISME J 7:1922–1932. doi:10.1038/ismej.2013.86
Shade A, Handelsman J (2012) Beyond the Venn diagram: the hunt for a core microbiome. Environ Microbiol 14:4–12. doi:10.1111/j.1462-2920.2011.02585.x
Horner-Devine MC, Lage M, Hughes JB, Bohannan BJ (2004) A taxa-area relationship for bacteria. Nature 432:750–753. doi:10.1038/nature03073
Bell T, Ager D, Song JI, Newman JA, Thompson IP, Lilley AK, van der Gast CJ (2005) Larger islands house more bacterial taxa. Science 308:1884. doi:10.1126/science.1111318
Acknowledgments
JT and SCF thank the MIT Center for Environmental Health Sciences (US National Institute of Environmental Health Sciences NIEHS grant P30-ES002109) MIT SeaGrant and MISTI-Brazil programs for funding. JT thanks Jonathan Friedman for helpful discussion regarding analysis of neutral community assembly. FLT and GG thank CNPq, FAPERJ, CAPES, and US Embassy for grants. We thank the ICOMM-LACAR project for 454 pyrosequencing.
Conflict of Interest
The authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Table S1
Water quality parameters for Sebastião Gomes (SG) and Parcel dos Abrolhos (PAB) reefs (XLS 23 kb)
Table S2
V6 tags removed using secondary structure alignment and delta G values (XLS 22 kb)
Table S3
RFLP screen of 16S rRNA clone libraries prepared from Mussismilia corals (XLS 22 kb)
Table S4
Taxonomic affiliation of cloned 16S rRNA gene sequences and corresponding V6 tag OTUs (XLS 41 kb)
Table S5
Permutational Analysis of Variance of microbiota structure from Mussismilia corals grouped by species and reef site with data filtered by occurrence of OTUs in multiple coral individuals (XLS 33 kb)
Table S6
Excel file with all OTUs, and abundances, with classification from RDP (XLS 2583 kb)
Fig. S1
Analysis of V6 secondary structure predictions to improve quality control. A) Distribution of ΔG’s for the most stable secondary structure of the 16S rRNA V6 region predicted using the CLC RNA Workbench secondary structure prediction tool (CLC bio USA, Cambridge, MA) with respect to frequency of reads in the current dataset (red) and the V6 reference database at VAMPS (blue). A ΔG value of −4.5 kcal/mol was identified as the minimum ΔG needed to form an acceptable secondary structure. 1.55 % of the reads were removed manually due to misalignment of secondary structure and/or anomalously high delta G values for the V6 stem-loop structure. B) Predicted secondary structures for representative 16S rRNA V6 OTUs. (JPEG 421 kb)
Fig. S2
Maximum likelihood tree of cloned and sequenced 16S rRNA genes from each coral species/reef. 16S rRNA from sequenced isolates were included for comparison (E. coli region from 515 to 1,300 nt). Sequences were clustered into ribotype groups at 97 % identity and a representative sequence was used for phylogenetic analysis. Type strains are indicated for phylogenetic comparison. Bootstrap values >50 % are indicated at branch nodes. Ribotype clusters containing sequences that match 16S V6 tag sequences from this study are highlighted in red and the corresponding V6 tag OTUs are indicated (JPEG 632 kb)
Fig. S3
Rarefaction analysis of each sample based on V6 OTUs generated at 97 % identity indicated that diversity was undersampled despite the deep-sequencing approach. The coral skeleton and sample PMHS2-3 had notably higher richness than other samples while PMBR2-1 and the white plague sample appeared to approach saturation. Rarefaction curves were generated using Mothur v.1.9.0. Abbreviations used: PMBR = M. braziliensis from Parcel dos Abrolhos reef, GMBR = M. braziliensis from Sebastiao Gomes reef, PMHS = M. hispida from Parcel dos Abrolhos reef, PMHA = M. harttii from Parcel dos Abrolhos reef (JPEG 352 kb)
Fig. S4
Identification of populations associated with healthy M. braziliensis. A) Distribution of 15 populations present in n = 6 M. braziliensis. B) The set of OTUs that was only observed in association with the healthy M. braziliensis samples may be enriched in populations that serve as bioindicators for a healthy holobiont. Thirty-six microbial OTUs satisfied the condition of being observed in the majority of healthy M. braziliensis samples (at least four of the six) but not observed in the skeleton or diseased sample. These OTUs may include stable associates of healthy coral tissue that are disrupted during disease. Heat maps indicate frequency of OTU sequences in the dataset. OTU abundances were standardized to 10,000 sequences per sample, subjected to a log(X + 1) transformation followed by addition of 1E-6 to each datapoint to eliminate zeros. Hierarchical clustering of OTUS was carried out in Multi Experiment Viewer (MeV) version 4.6 implementing the Pearson correlation and average linkage method. Taxonomic assignments are based on RDP classifier (bootstraps in brackets) or by match to a cloned 16S rRNA sequence (nucleotide identity given in %) (JPEG 1010 kb)
Fig. S5
Comparison of the distribution of OTUs in 12 healthy Mussismilia corals to a model of neutral community assembly. A) The mean relative abundance of HC-OTUs and the frequency with which the 7,525 HC-OTUs appear among the 12 healthy Mussismilia corals (i.e., prevalence) are plotted for observed data (points) and modeled data (line) based on fitting parameter mN as calculated in Sloan et al. (2006). B) Distribution of R^2 values obtained when fitting 50 simulated communities generated with the fitted parameter mN. The simulated datasets fit the neutral model significantly better than the observed data (p < 0.02); thus, a locally neutral community assembly model can be rejected for our dataset (JPEG 313 kb)
Fig. S6
The average richness of bacterial OTUs (S) in increasing numbers of Mussismilia coral samples was calculated from (i) the total OTU abundance or from OTU abundance in the dataset downsampled without replacement to 583 sequences. Species accumulation was calculated using the species accumulation function in Primer6 with 1,000 permutations considering all coral samples (n = 12) (open symbols) or restricted to M. braziliensis corals only (n = 6) (crossed symbols). A linear relationship was observed between log-transformed estimates of OTUs observed and number of coral samples under all conditions (R^2 > 0.998). Based on this regression, the exponent (z) was 0.82 to 0.85 (JPEG 344 kb)
Rights and permissions
About this article
Cite this article
Fernando, S.C., Wang, J., Sparling, K. et al. Microbiota of the Major South Atlantic Reef Building Coral Mussismilia . Microb Ecol 69, 267–280 (2015). https://doi.org/10.1007/s00248-014-0474-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-014-0474-6