Abstract
Background
The process of vascular development is essential for shaping complex craniofacial structures. Investigating the interplay between vascular development and orofacial morphogenesis holds critical importance in clinical practice and contributes to advancing our comprehension of (vascular) developmental biology. New insights into specific vascular developmental pathways will have far-reaching implications across various medical disciplines, enhancing clinical understanding, refining surgical techniques, and elucidating the origins of congenital abnormalities. Embryonic development of the craniofacial vasculature remains, however, under-exposed in the current literature. We imaged and created 3-dimensional (D) reconstructed images of the craniofacial arterial system from two early-stage human embryonic samples.
Objective
The aim of this study was to investigate the vascular development of the craniofacial region in early-stage human embryos, with a focus on understanding the interplay between vascular development and orofacial morphogenesis.
Materials and methods
Reconstructions (3-D) were generated from high-resolution diffusible iodine-based contrast-enhanced computed tomography (diceCT) images, enabling visualization of the orofacial arterial system in human embryonic samples of Carnegie stages (CS) 14 and 18 from the Dutch Fetal Biobank, corresponding to weeks 7 and 8.5 of gestation.
Results
From two human embryonic samples (ages CS 14 and 18), the vascular development of the orofacial region at two different stages of development was successfully stained with B-Lugol and imaged using a micro-computed tomography (micro-CT) scanner with resolutions of 2.5-μm and 9-μm voxel sizes, respectively. Additionally, educational 3-D reconstructions of the orofacial vascular system were generated using AMIRA 2021.2 software.
Conclusion
Micro-CT imaging is an effective strategy for high-resolution visualization of vascular development of the orofacial region in human embryonic samples. The generated interactive 3-D educational models facilitate better understanding of the development of orofacial structures.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The complex process of vascular development plays a pivotal role in shaping the intricate craniofacial structures [1]. Proper vascular development is paramount for normal growth and maturation of tissues [2, 3]. In comparison with the extensive research conducted in the realm of neurovascular development, our understanding of orofacial vascular development remains limited. Orofacial malformations, such as craniofacial synostoses, cleft lip and cleft palate, micrognathia, and macroglossia, pose significant challenges in clinical practice [4]. Despite their prevalence and clinical significance, the etiology of many of these malformations remains elusive. This knowledge gap underscores the need to delve deeper into the role of vascular development in the genesis of craniofacial malformations. By unraveling the complexities of craniofacial vascular development, we aim to shed light on the underlying mechanisms contributing to a spectrum of craniofacial malformations [1].
Historical perspective of imaging human development
Traditionally, the majority of developmental research focused on visualizing and describing vascular development relied on histological sections of embryologic specimens for analysis and reconstruction. In this technique, individual surfaces, obtained from these sections, generated sequentially using a microtome, were reconstructed to form a 3-dimensional (D) visualization [5]. This technique might be considered the gold standard for visualizing embryological development. Wilhelm His, a prominent anatomist and embryologist (1831–1904), adopted this method in his embryological research [6]. Initially focusing on chick embryos, he later applied microtome techniques to human embryos, producing histological stained tissue sections for reconstructions of, what Wilhelm His named, “plastic views” of entire embryos or specific organs [6]. His work introduced human specimens to the research of embryological development during the late nineteenth century. Employing samples derived from hysterectomies, ectopic pregnancies, and spontaneous abortions, obtained through a network of medical professionals, he aimed to standardize developmental depictions of human embryos, which were then termed “normal plates” [7, 8]. More recently, Rana et al. [9] created a 3-D reference model from the aortic arch development using a digital version of the similar techniques. Recently, Belle et al. [10] published their remarkable study employing light sheath microscopy as a modality for imaging and 3-D visualization of detailed human embryological development at various stages. For imaging vascular development, light sheath microscopy has been proven as an outstanding technique, providing excellent details and 3-D visualization of the developing vascular tree [11, 12]. In addition, Ramirez et al. [12] showed promising results visualizing and analyzing pharyngeal arch arteries with an immunohistochemistry technique and 3-D reconstructions. However, a great disadvantage of using these techniques on precious embryonic samples is their destructive character.
Fortunately, new technological modalities have been developed, such as computed tomography (CT), various microscopy techniques, and ultrasound, capable of visualizing structures and making 3-D reconstructions without destructing the original sample. In recent years, it is demonstrated that advancements in imaging techniques have revolutionized our ability to investigate intricate processes of embryonic development [10, 13]. The use of micro-CT imaging of human embryonic samples has emerged as a powerful tool for visualizing and analyzing the dynamic changes in vascular networks during orofacial development. This imaging modality allows for high-resolution, 3-D reconstruction of vascular structures, providing unprecedented insights into the spatial and temporal dynamics of vascular morphogenesis of the orofacial region [13, 14].
Vascular development
The vascular development of the external carotid system starts with the initiation of the primary system for the extra cranial arteries at Carnegie stage (CS) 8 and finalizes at CS 23 [15, 16]. The majority of the vascular system, especially the major blood vessels of the body and the intracranial region, originates through vasculogenesis, a process involving the de novo formation of blood vessels [17]. In contrast, the development of extracranial vessels does primarily occur through angiogenesis, a process driven by hypoxia, and characterized by the emergence of new blood vessels through sprouting and branching from pre-existing ones [18, 19].
The primary aim of this study is to investigate the craniofacial vascular development in human embryos. To achieve this, we provide a comprehensive understanding on embryological development of the craniofacial arterial development. Additionally, we aim to produce educational 3-D reconstructions that will facilitate the comparison of our findings on early-staged craniofacial vascular development with the reviewed literature. By integrating these detailed reconstructions with the current body of knowledge, we hope to enhance the comprehension of craniofacial vascular anatomy, particularly focusing on the external carotid system. Such an enriched understanding is crucial for accelerating the identification of etiological factors underlying craniofacial birth defects.
Methods
Embryonic samples
Sample acquisition
In this study, two human embryonic samples were used: one at CS 14 (TOP 174, 4-mm crown-rump length (CRL)) and the other at CS 18 (TOP 318, 15-mm CRL), corresponding to gestational ages of 7 weeks and 8.5 weeks, respectively. These specific samples were included due to their availability from the Dutch Fetal Biobank [14]. The specimens were donated with parental written informed consent after removal of an ectopic pregnancy. Ethical approval of the Dutch Fetal Biobank was granted by the Medical Ethical Committee (METC) of the Amsterdam University Medical Center, location AMC, Amsterdam, The Netherlands (METC 2016_285).
Embryo sample preparation and scanning protocol (Fig. 1)
After surgical resection of the fallopian tube, samples were fixed in 4% paraformaldehyde solution for 48 h, and then stored at 4 °C in a 0.2% paraformaldehyde solution. A diffusible iodine-based contrast-enhanced computed tomography (diceCT) staining technique for imaging of the human embryonic soft tissues was used [13, 14], prior to exposure of the embryo to the micro-CT scan. Staining was performed by submerging the sample for 5 days in a 3.75% buffered Lugol’s solution [20]. Apart from its general contrast-enhancement of tissues, this solution typically enhances the visibility of blood and vessels on micro-CT scans due to its high affinity with blood. Micro-CT scans were performed using a UniTOM XL micro-CT unit (TESCAN XRE Demo Lab, Ghent, Belgium) providing images with a voxel size for samples 1 (CS 14) and 2 (CS 18) of 2.5 μm (scan settings 80 kV, 15 W and scan time 7 h 42 min) and 9.0 μm (scan settings 100 kV, 15 W and scan time 2 h 16 min), respectively.
Data visualization
The software package Amira 3-D (version 2021.2, Thermo Fisher Scientific, MA, USA) was used for the creation of 3-D reconstructions of the intra- and extracranial vascular system [21]. Micro-CT data sets from both human embryonic samples (CS 14 and 18) were used for annotation and segmentation. The reconstructions were mostly manually segmented with a Bamboo tablet and stylus (http://www.wacom.com). After generation of the 3-D models, surfaces were smoothed, without loss of essential details. Labels were assigned to the different anatomical structures after the 3-D reconstructions were completed (Fig. 1). Segmentation and labeling were accomplished by two trained dentistry students, under the supervision of the first author.
Supplementary 3-D-PDFs containing the 3-D reconstructions of both stages were added to the online version of this article and can be viewed with a recent version of Adobe Reader (free-ware available at http://www.adobe.com).
Terminology
Weeks/days
Gestational age, calculated from the first day of the last menstrual period, is commonly used to denote the clinical age of an embryo or fetus, while post-conception age refers to the number of days elapsed since fertilization. Throughout our study, we will refer to “days” or “weeks” as gestational age.
CS
The Carnegie stages, proposed by Streeter in 1942 and later revised by O’Rahilly and Müller in 1987 and 2010 [22], are based on the observed external features of developing human embryos in the first 60 days of their development post-fertilization.
Background
The development of the craniofacial arterial system begins with the genesis of five pairs of primitive pharyngeal arch arteries, which undergo significant modifications throughout development [9, 15, 23,24,25,26,27]. The fundamental organization of the pharyngeal regions arises with the emergence of pharyngeal pouches, marking a crucial event in the development of the pharyngeal arches [23, 24]. Each pharyngeal arch contains various embryonic cell types, including ectoderm (external), endoderm (internal), and a mesenchymal filling of the neural crest with a central core of mesoderm [23]. These distinct embryonic cell populations contribute to different components of the arch: the ectoderm forms the epidermis and sensory neurons of the epibranchial ganglia; the endoderm gives rise to the epithelial lining of the pharynx, taste buds, thyroid, parathyroid, and thymus; the neural crest in this layer forms the skeletal and connective tissues of the arches; and the mesoderm contributes to the musculature and endothelial cells [23, 28].
During CS 11 to 20, the intracranial vascular system, including the circle of Willis, is formed. This process has been thoroughly studied and documented in the literature [16, 26, 27, 29], particularly due to its clinical significance and the association between congenital arterial malformations in intracranial vessels and the risk of cerebrovascular pathology later in life [30, 31].
Older pioneers in vascular development, as noted by Bertulli and Robert [15], have focused more on the formation of the extracranial arterial system. Notably, several studies by Streeter, Congdon, and Padget [16, 32,33,34] utilized the Carnegie embryo collection to outline the foundational principles of craniofacial vascular system formation. Additionally, significant contributions have been made by Altmann and Lasjaunias [35,36,37], who provided research on arterial variants, comparative anatomy, neurovascular anatomy, and embryology.
The development of the extracranial arterial system commences at CS 8, with the production of mesodermal substrate during the process of gastrulation. Subsequently, various modification and formation processes occur throughout embryonic and fetal development, ultimately culminating in the formation of the extracranial vascular system. Table 1 provides a schematic overview of these developmental stages.
Each of the pharyngeal arteries will differentiate, modulate, and finally participate in the development of specific parts of the extracranial vascular system and vascularize the orofacial and extracranial regions. The bilateral present external carotid artery (ECA) will be the basis of the extracranial vessels. Each of these vessels will find their origin in one of four different pharyngeal arches (1st–4th), with most of them deriving from the 2nd pharyngeal arch (see Fig. 2 with corresponding Table 2 for an overview of ECA branches and the pharyngeal artery they derived from).
Graphical comprehensive overview of all arterial branches, their derivatives, and the corresponding pharyngeal arches of which they originate from, detailing the oxygenated blood supply to the orofacial and extracranial regions: 1st arch derivatives (dark red), 2nd arch artery derivatives (orange), 3rd arch artery derivatives (green), 4th arch derivatives (blue). CCA common carotid artery, ECA external carotid artery, ICA internal carotid artery (bright red). Details of each of these arteries are provided in Table 2 (Figure adapted from Cartens et al. and Thieme/Prometheus 2010 [38, 39])
Results
Reconstruction embryonic sample 1, CS 14
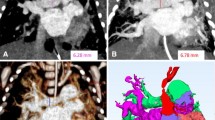
When examining our 3-D reconstruction of a human embryonic specimen at CS 14, several unique observations were made. Our reconstruction revealed numerous cardiovascular structures, including the posterior cerebral artery (PCA stem), basilar artery (BA), internal carotid artery (ICA), third pharyngeal arch artery (AA3), fourth pharyngeal arch artery (AA4), aortic sac (AS), and dorsal artery (DA). Clearly in this stage of development, we have not been able to annotate and reconstruct all arterial structures. Some arterial structures that according to literature should have been visible were not present or visible in our data set (Table 3). The third and fourth pharyngeal arches are perceptible and bilaterally connected to the aortic sac. Figure 3 presents the cardiovascular anatomy from our reconstruction in situ, and Table 3 demonstrates an overview of the cardiovascular structures presented in our reconstruction compared with literature.
Three-dimensional reconstruction at Carnegie stage 14 (4-mm crown-rump length). a In situ 3-dimensional reconstruction of the intra- and extracranial vascular system. b Vascular tree 3-dimensional reconstructions of the intra- and extracranial vascular system. Panels (a) and (b) were both generated from micro-computed tomography contrast-enhanced high-resolution images (2.5-µm voxel size); the structures shown are listed in Table 3. AA3 third pharyngeal arch artery, AA4 fourth pharyngeal arch artery, AS aortic sac, BA basilar artery, DA dorsal aorta, ICA internal carotid artery, PCA stem posterior cerebral artery
Reconstruction embryonic sample 2, CS 18
During our examination of the 3-D reconstruction of a human embryonic specimen at CS 18, we identified several significant anatomical features. The posterior inferior cerebellar arteries and the spinal arteries were nicely visible. Moreover, our reconstruction displayed detailed branches from the hyoid artery and the anterior choroidal artery. The external carotid artery was also well-represented, showing multiple vascular branches. Furthermore, we demonstrate various vascular structures in detail. The vertebral arteries displayed a noticeable zig-zag configuration, the basilar artery showed intricate side branches, and the primitive central ophthalmic artery extended to the eye. Certain structures were exclusively discernible on the left side of the embryo, including the diencephalic artery, the mesencephalic artery, and a part of the external carotid artery. However, some cardiovascular structures were not observable in our reconstruction. These included the persistent trigeminal artery, the ascending aortae, the pulmonary trunk, and the pulmonary arteries.
Figure 4 presents the cardiovascular anatomy from our reconstruction, schematic and in situ, and Table 3 demonstrates an overview of the cardiovascular structures presented in our reconstruction compared with literature.
Three-dimensional reconstruction at Carnegie stage 18 (15-mm crown-rump length). a In situ 3-dimensional reconstruction of the intra- and extracranial vascular system. b Vascular tree 3-dimensional reconstructions of the intra- and extracranial vascular system. Panels (a) and (b) were both generated from micro-computed tomography contrast-enhanced high-resolution images (9.0-µm voxel size); the structures shown are listed in Table 3. ACA anterior cerebral artery, AChA anterior choroidal artery, AICA anterior inferior cerebral artery, AS aortic sac, BA basilar artery, CCA common carotid artery, DA dorsal aorta, ECA external carotid artery, HyA hyoid artery, ICA internal carotid artery, MCA middle cerebral artery, PCA stem posterior cerebral artery, PChA posterior choroidal artery, Pcom posterior communicating artery, PICA posterior inferior cerebral artery, P.olf.A primitive olfactory artery, PVOA primitive ventral ophthalmic artery, SpA spinal artery, VA vertebral artery
Discussion
Our aim was to investigate the craniofacial vascular development in early-stage human embryos, with a particular focus on extracranial arteries. This topic has been underrepresented in current literature, despite the critical importance of early embryonic stages in laying the foundation for eventual anatomy. Through this research, we hope to contribute to a better understanding of congenital anomalies in the craniofacial region, such as craniofacial synostoses, cleft lip and cleft palate, micrognathia, and macroglossia [2,3,4].
This study marks the first instance of non-destructive micro-CT imaging being used to chart the craniofacial structures of early human embryos. Generally, our results align with sources that describe embryonic development via histology [27, 41]. Specifically, in CS 18, we delved deeper into detailing the branches of intra- and extracranial arteries through the creation of 3-D reconstructions.
For educational purposes, we have rendered the 3-D reconstructions of the two embryos from this pilot study into 3-D-PDF format (supplementary materials). This allows readers to interactively explore the models, identifying and toggling individual structures, enhancing students’ 3-D understanding of complex vascular structures [42].
Unique embryonic samples
The Dutch Fetal Biobank, established in 2017 at Amsterdam UMC, is a unique resource for embryonic and fetal tissue [43]. Its uniqueness stems from three factors: the liberal political climate in The Netherlands essential for such a resource’s existence, high parental willingness to donate to science, and the biobank team’s round-the-clock readiness to collect donations, ensuring tissue is included within hours of birth. Additionally, unlike many countries where pregnancies are terminated via curettage, The Netherlands increasingly prefers medical terminations due to the adverse effects of curettage on future pregnancies [44]. This preference ensures we receive intact specimens, facilitating thorough anatomical research.
The two embryos used in this study were obtained through the surgical removal of a fallopian tube in ectopic pregnancies, where the pregnancy implants in a non-uterine location, posing a life-threatening risk to the mother. A question arises whether this abnormal location impacts the embryo’s development, potentially due to suboptimal placental development [45]. However, our experience in studying human embryos from ectopic pregnancies indicates their development aligns with the known Carnegie staging, and their anatomy is comparable to intrauterine embryos. The impressive vascularization around the fallopian tube may ensure adequate placental development during early stages. Despite our experience and the similarities in Carnegie staging and anatomical comparability, we must acknowledge that there is no absolute certainty that extrauterine and intrauterine developments are identical. Therefore, we must be cautious when interpreting data obtained from ectopic pregnancies, as it may not be equivalent to that from intrauterine embryonic development.
This pilot study’s inclusion of only two human embryos is insufficient to draw conclusions about anatomical variation. Early-stage human embryonic samples are extremely rare, but with the growing number of samples in the Dutch Fetal Biobank, we aim to provide a comprehensive overview of craniofacial vascular development. We hypothesize that alterations in vascular development can negatively impact normal growth and development, potentially contributing to orofacial malformations. Further research is needed to substantiate the role of vascular development as an etiological factor for these malformations. Understanding the normal development of cranial arteries will enable comparisons with aberrant developmental patterns in embryos with congenital anomalies currently being prospectively collected in the biobank.
Micro-CT imaging as a method to study vasculature
Historically, mapping craniofacial arteries in human embryos required destructive methods, including histology, immunohistochemistry, and light sheath microscopy [9, 10, 16]. Given the scarcity of complete and healthy human embryos available for scientific research, it is imperative to handle these specimens with the utmost respect, ideally preserving them intact. Our experience with contrast-enhanced micro-CT imaging using buffered Lugol’s solution to prevent extensive shrinkage (ranging from 15 to 35% for lung, brain, liver, or total body volume when using non-buffered Lugol’s solution) has enabled us to scan embryos obtained from ectopic pregnancies while maintaining the integrity of the amniotic sac, at resolutions of 2.5-μm or 9-μm voxel size. For perspective, the average human hair is 70 μm thick. In this pilot study, we presented results from the first two human embryos mapped using micro-CT imaging.
Our findings revealed that vascular structures in our models sometimes lacked bilaterality, contrary to expectations for these developmental stages. A plausible explanation, supported by histological studies of human embryos [27, 41], is the post-mortem redistribution of blood cells to the lowest side when an embryo is laid down. This phenomenon which is also known from adult post-mortem cases likely occurs before fixation, making vessels on the lower side appear fuller than those on the higher side. The higher side’s small vessels sometimes seem “empty” as they contain no red blood cells. Combined with iodine contrast’s high affinity for blood, this makes reconstructing vessels on the higher side challenging or impossible.
Another challenge of our method is the manual segmentation required for annotating all vessels in micro-CT images. This is an established method for studying anatomical structures in 3-D, with low inter-observer variability when performed by trained yet unbiased students under experienced embryologists’ supervision [41]. The relative standard error in such manual reconstructions ranges from only 0.3% for simple structures to 2% for complex ones such as blood vessels [27]. However, manual segmentation is time-intensive, and advances in artificial intelligence may soon offer solutions to this issue [5].
Conclusion
Our study presents the first non-destructive mapping of craniofacial arteries in early-staged human embryos using micro-CT imaging, revealing valuable insights into vascular development and providing educational models. Although limited by the small sample size, our findings lay the groundwork for future research with larger cohorts from the Dutch Fetal Biobank, ultimately advancing our understanding of normal and abnormal craniofacial vascular development.
Data availability
The data that support the findings of this study are available from the corresponding author, K.J., upon reasonable request.
References
Sun MR, Chung HM, Matsuk V et al (2020) Sonic hedgehog signaling in cranial neural crest cells regulates microvascular morphogenesis in facial development. Front Cell Dev Biol 8:590539
Franz-Odendaal TA, Andrews D, Kumar S (2017) Angiogenesis meets skeletogenesis: the cross-talk between two dynamic systems. Physiologic and pathologic angiogenesis - signaling mechanisms and targeted therapy. InTec. https://doi.org/10.5772/66497
Filipowska F, Tomaszewski KA, Niedźwiedzki Ł et al (2017) The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 20:291–302
Agbenorku P (2013) Orofacial clefts: a worldwide review of the problem. ISRN Plast Surg 2013:1–7
Dawood Y, Buijtendijk MFJ, Shah H et al (2022) Imaging fetal anatomy. Semin Cell Dev Biol 131:78–92
His W (1870) Beschreibung eines Mikrotoms. Arch Mikrosk Anat 6:229–232
Hopwood N (2000) Producing development: the anatomy of human embryos and the norms of Wilhelm His. Bull Hist of Med 74:29–79
Hopwood N (2007) A history of normal plates, tables and stages in vertebrate embryology. Int J Dev Biol 51:1–26
Rana MS, Sizarov A, Christoffels VM, Moorman AFM (2014) Development of the human aortic arch system captured in an interactive three-dimensional reference model. Am J Med Genet A 164:1372–1383
Belle M, Godefroy D, Couly G et al (2017) Tridimensional visualization and analysis of early human development. Cell 169:161–173
Blain R, Couly G, Shotar E et al (2023) A tridimensional atlas of the developing human head. Cell 186:5910–5924
Ramirez A, Astrof S (2020) Visualization and analysis of pharyngeal arch arteries using whole-mount immunohistochemistry and 3d reconstruction. J Vis Exp 2020:157
Docter D, Dawood Y, Jacobs K et al (2023) Microfocus computed tomography for fetal postmortem imaging: an overview. Pediatr Radiol 53:632–639
Dawood Y, Strijkers GJ, Limpens J et al (2020) Novel imaging techniques to study postmortem human fetal anatomy: a systematic review on microfocus-CT and ultra-high-field MRI. Eur Radiol 30:2280–2292
Bertulli L, Robert T (2021) Embryological development of the human cranio-facial arterial system: a pictorial review. Surg Radiol Anat 43:961–973
Padget DH (1948) The Development of the Cranial Arteries. 212:205–262
Patan S (2000) Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neurooncol 50:1–15
Klostranec JM, Krings T (2022) Cerebral neurovascular embryology, anatomic variations, and congenital brain arteriovenous lesions. J NeuroInterv Surg 14:910–919
Potente M, Mäkinen T (2017) Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol 18:477–494
Dawood Y, Hagoort J, Siadari BA et al (2021) Reducing soft-tissue shrinkage artefacts caused by staining with Lugol’s solution. Sci Rep 11:19781
https://www.thermofisher.com/nl/en/home/electron-microscopy/products/software-em-3d-vis/3d-visualization-analysis-software/whats-new.html. “Thermofisher.com.”
O’Rahilly R, Müller F (2010) Developmental stages in human embryos: revised and new measurements. Cells Tissues Organs 192:73–84. https://doi.org/10.1159/000289817
Graham A (2001) The development and evolution of the pharyngeal arches. J Anat 199:133–141
Graham A, Hikspoors JPJM, Anderson RH et al (2023) A revised terminology for the pharyngeal arches and the arch arteries. J Anat 243:564–569
Rodríguez-Vázquez JF, Mérida-Velasco JR, Verdugo-López S et al (2006) Morphogenesis of the second pharyngeal arch cartilage (Reichert’s cartilage) in human embryos. J Anat 208:179–189
Anderson RH, Bamforth SD (2022) Morphogenesis of the mammalian aortic arch arteries. Front Cell Devl Biol 10:892900
De Bakker BS, de Jong KH, Hagoort J et al (2016) An interactive three-dimensional digital atlas and quantitative database of human development. Science 354:aag0053
Shone V, Graham A (2014) Endodermal/ectodermal interfaces during pharyngeal segmentation in vertebrates. J Anat 225:479–491
Congdon ED (1922) Transformation of the aortic-arch system during the development of the human embryo. In: Contribution to Embryology, Carnegie Institution Washington, vol 277, pp 47–110
Oumer M, Alemayehu M, Muche A (2021) Association between circle of Willis and ischemic stroke: a systematic review and meta-analysis. BMC Neurosci 22:3
van Seeters T, Hendrikse J, Biessels GJ et al (2015) Completeness of the circle of Willis and risk of ischemic stroke in patients without cerebrovascular disease. Neuroradiology 57:1247–1251
Congdon ED (1922) Transformation of the branchial-arch system during the development of the human embryo. Contrib Embryol 68:47–110
Padget DH (1956) The cranial venous system in man in reference to development, adult configuration, and relation to the arteries. Am J Anat 98:307–355
Streeter GL (1942) Developmental horizons in human embryos. Contrib Embryol 541:211–245
Altmann F (1947) Anomalies of the internal carotid artery and its branches; their embryological and comparative anatomical significance; report of a new case of persistent stapedial artery in man. Laryngoscope 57:313–339
Lasjaunias P (2001) Surgical neuroangiography, 2nd edn., vol. 1. Springer, Berlin, Heidelberg, New York
Lasjaunias P (2003) The development and evolution of vascular malformations. Rivista Neuroradiologia 16 - sup 2. https://doi.org/10.1177/1971400903016SP204
Carstens HM (2023) The embryologic basis of craniofacial structure. Springer International Publishing, Saint Louis. https://doi.org/10.1007/978-3-031-15636-6
Schuenke M, Schulte E, Schumacher U (2010) Atlas of anatomy. In: Head and neuroanatomy. THIEME Stuttgart, New York, p 420
Standring S (2016) Grays’s anatomy, 42nd edn. Elsevier, London England
De Bakker BS, de Jong KH, Hagoort J et al (2012) Towards a 3-dimensional atlas of the developing human embryo: the Amsterdam experience. Reprod Toxicol 34:225–236
Chekrouni N, Kleipool RP, De Bakker BS (2020) The impact of using three-dimensional digital models of human embryos in the biomedical curriculum. Ann Anat 227:151430
Dawood Y, Buijtendijk MFJ, Bohly D et al (2023) Human embryonic and fetal biobanking: establishing the Dutch Fetal Biobank and a framework for standardization. Dev Cell 58:2826–2835
Saccone G, Perriera L, Berghella V (2016) Prior uterine evacuation of pregnancy as independent risk factor for preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol 214:572–591
Woods L, Perez-Garcia V, Hemberger M (2018) Regulation of placental development and its impact on fetal growth—new insights from mouse models. Front Endocrinol (Lausanne) 9:570
Author information
Authors and Affiliations
Contributions
K. J. contributed to the design and implementation of the research, to the analysis of the results, and to the writing of the manuscript with support from G.E.J.L., D.D., P.A.M.C., B.J.B., J.A.M.K., S.C.V., J.J.P., J.H., F.L., and B.S.B. who supervised the project. Micro-CT research on human embryonic samples was performed by K.J., D.D., and B.S.B. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and informed consent
Specimens were donated with parental written informed consent after a medically induced termination of pregnancy, removal of an ectopic pregnancy, or preterm delivery. Ethical approval of the Dutch Fetal Biobank (DFB) was granted by the Medical Ethical Committee (METC) of the Amsterdam University Medical Centre, location AMC, Amsterdam, The Netherlands (METC-2016_285).
Conflicts of interest
None
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jacobs, K., Langenbach, G.E.J., Docter, D. et al. Imaging the development of the human craniofacial arterial system – an experimental study. Pediatr Radiol (2024). https://doi.org/10.1007/s00247-024-06044-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00247-024-06044-x