Abstract
Background
One of the main limitations of 99mtechnetium-dimercaptosuccinic acid (DMSA) scan is the long acquisition time.
Objective
To evaluate the feasibility of short DMSA scan acquisition times using a cadmium-zinc-telluride-based single-photon emission computed tomography (SPECT) system in children.
Materials and methods
The data of 27 children (median age: 4 years; 16 girls) who underwent DMSA SPECT were retrospectively analyzed. Both planar and SPECT DMSA were performed. SPECT images were analyzed using coronal-simulated planar two-dimensional images. A reduction in SPECT acquisition time was simulated to provide 4 series (SPECT-15 min, SPECT-10 min, SPECT-5 min and SPECT-2.5 min). A direct comparison of the planar and SPECT series was performed, including semi-quantification reproducibility, image quality (mean quality score on a scale of 0 to 2) and inter- and intra-observer reproducibility of the scintigraphic patterns.
Results
The overall image quality score (± standard deviation) was 1.3 (± 0.6) for the planar data set, 1.6 (± 0.5) for the SPECT-15 min data set, 1.4 (± 0.5) for the SPECT-10 min data set, 1.0 (± 0.5) for the SPECT-5 min data set and 0.6 (± 0.6) for the SPECT-2.5 min data set. Median Kappa coefficients for inter-observer agreement between planar and SPECT images were greater than 0.83 for all series and all readers except one reader for the SPECT-2.5 min series (median Kappa coefficient = 0.77).
Conclusion
Shortening SPECT acquisitions to 5 min is feasible with minimal impact on images in terms of quality and reproducibility.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Major advances in nuclear medicine both in the fields of instrumentation and image reconstruction have been reported over the last decade. This progress has translated into shorter scanning time and/or reduced amount of radiopharmaceutical activity administered without loss of image quality compared to conventional techniques. To date, these efforts have focused on positron emission tomography (PET) scans and conventional nuclear medicine, mostly for myocardial investigations [1,2,3]; little has been done for renal scintigraphy, even though these exams mainly concern pediatric patients who would benefit from shorter scans [4,5,6].
The 99mtechnetium-dimercaptosuccinic acid (DMSA) scan is the reference method for imaging the renal cortex and to quantify split renal function [7]. In children, common indications include detection of renal sequelae of pyelonephritis and estimation of functioning renal mass [8]. However, one of the main limitations of DMSA scans is a long acquisition time (15–30 min) [9]. This is particularly important when used in children, who are more prone to move during the acquisition.
Gamma cameras with cadmium-zinc-telluride (CZT) semiconductor detectors have the potential to reduce acquisition times or the injected activity because of their improved contrast and energy resolution [10]. Several studies have shown that CZT single-photon emission computed tomography (SPECT), designed for cardiac imaging, is ultrafast [1]. As such, whole-body SPECT scanning with CZT semiconductor detectors has become commercially available [11]. Although this scanner has the potential to reduce scan times, its clinical efficacy remains to be demonstrated for short DMSA scan acquisition times.
Our hypothesis was that the SPECT CZT gamma camera allows considerable reduction of DMSA scan times, without increasing the radiation dose. The aim of this study was to determine to what extent such reduction is possible in children without losing clinical information.
Materials and methods
This retrospective study was approved by our institutional review board and informed consent was waived because of its retrospective nature. From October 2016 to March 2017, all children referred for a DMSA scan in our institution were consecutively included.
Image acquisition
The administered activity was based on patient weight according to the European Association of Nuclear Medicine pediatric dosage card [12]; the median activity was 27 (25–42) MBq (0.73 mCi [0.68–1.14]).
Planar images were acquired 2–3 h after radiopharmaceutical injection using a double head camera Discovery NM/CT CZT 670 (GE Healthcare, Haifa, Israel). Images included anterior, posterior, left anterior oblique, right anterior oblique, left posterior oblique and right posterior oblique projections. Each image required at least 300,000 counts and were reconstructed using a 128 × 128 matrix. In total, planar image acquisition took 30 min per patient.
Immediately thereafter, SPECT was performed using the same gamma-camera. SPECT acquisition, set in body contour mode, comprised 60 projections (30 steps with 6° rotation angle using the dual head gamma detector) of 30 s for an effective 15-min scan time. The raw data were further reconstructed as full-time (30 s/projection: SPECT-15 min) or truncated (20 s/projection, resulting in a simulated 10-min acquisition: SPECT-10 min; 10 s/projection, resulting in a simulated 5-min acquisition: SPECT-5 min and 5 s/projection, resulting in a simulated 2.5-min acquisition: SPECT-2.5 min) time series using Lister software (GE Healthcare). All series were reconstructed and processed under the same conditions using an ordered subset expectation maximization (OSEM: two iterations, 10 subsets), a Butterworth post-filter (0.6 cutoff, factor 10) and a matrix of 128 × 128.
Semi-quantification and qualitative assessment
SPECT images were analyzed using coronal-simulated planar two-dimensional (D) images. This method is known to be simple and effective for analyzing DMSA SPECT images [13].
Semi-quantitative analyses to evaluate the renal split function were performed on planar and each series of the SPECT images using Xeleris Workstation 3.1™ (GE Healthcare) that automatically placed regions of interest (ROI) for the entire kidney and background noise under visual control of two operators (B.C., a nuclear medicine fellow with 6 months of experience and M.J., a nuclear medicine physician with > 20 years of experience).
After anonymization and randomization, 3 experienced nuclear medicine physicians (B.G., G.P.J. and C.M.T., with 6, 13 and 12 years of experience, respectively) who regularly read nuclear medicine studies independently reviewed planar and SPECT images, using a grayscale image as recommended [4], blinded to the clinical data and image characteristics. All readers reviewed all the series. A qualitative evaluation of the contrast, resolution and the resulting image quality scores was performed according to the scoring scales and the definitions presented in Table 1. Examples of image quality scores rated as 0, 1 and 2 are presented in Fig. 1. The image quality scores for each and for all reviewers were collected.
Anterior 99mtechnetium-dimercaptosuccinic acid (DMSA) images of the kidneys illustrating image quality scores. a A score of 0 in an 11-month-old girl with bilateral duplex kidneys and recurrent febrile urinary tract infections (UTIs). On the DMSA images, there is evidence of a left duplex kidney (arrowhead depicting the cortical bar) and diffuse volume loss of the right lower moiety (asterisk) that is probably developmental (reflux nephropathy). b A score of 1 in a 7-year-old girl with recurrent UTIs. A small scar is seen in the upper pole of the left kidney (arrow). c A score of 2 in a 4-year-old boy with recurrent UTIs, who had a normal DMSA scan on presentation
Inter- and intra-observer agreement of the following scintigraphic findings were evaluated: renal size (symmetrical or asymmetrical) and renal function (symmetrical, decreased right or left renal functioning mass or absent uptake throughout the entire right or left kidney); and for each kidney, contour (preserved or irregular) and cortical uptake (preserved, or single/multiple low or no signal area). To assess intra-observer reproducibility, each series was read twice in random order after a three-month interval.
Continuous data are expressed as mean ± standard deviation (SD) or median [interquartile range] and categorical data as a percentage. The paired sample t-test was used to compare the differences in continuous variables between the planar and SPECT series. Bland–Altman plots were generated for each dataset, taking planar images as reference. Inter- and intra-observer reproducibility was assessed using Kappa coefficients. According to Landis and Koch guidelines, Kappa values of 0.21 to 0.40 indicate fair agreement, 0.41 to 0.60 indicate moderate agreement, 0.61 to 0.80 indicate substantial agreement and 0.81 to 1.0 indicate almost perfect or perfect agreement. A statistically significant difference was defined as P < 0.05. All statistical analyses were conducted using R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
During the study period, 41 patients underwent DMSA scan. Due to technical problems related to the list-mode recording (list-mode data not available, n = 5), because of single kidney (non-evaluation of relative renal function, n = 2) or due to being 18 years of age or older (n = 7), 14 patients were excluded from subsequent analyses. The remaining 27 children were included in the analysis. The median age was 4 years [0.95–8]; 17 (63%) were 5 years of age or younger; 16 children (59%) were girls and the median body weight was 14 kg [10–26 kg]. No child was sedated.
Patients were referred for a DMSA scan to exclude renal scars following a febrile urinary tract infection (44%), because of vesico-ureteral reflux (30%) or to estimate relative functioning renal mass (26%). Examples of planar and 2-D coronal projection SPECT images for a boy with a normal DMSA scan (Fig. 2) and for a girl with a renal scar (Fig. 3) are presented.
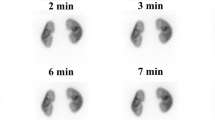
Comparison of planar and single-photon emission computed tomography (SPECT) images in a 1-year-old boy who was referred for a 99mtechnetium-dimercaptosuccinicacid (DMSA) examination to exclude renal parenchymal scarring following an acute urinary tract infection. a–l Conventional planar images (a–f) and 5-min scan two-dimensional coronal projection SPECT images (g–l), showing homogeneous and diffuse cortical renal absorption. Renal function was estimated to be 52% and 48% for the right and left kidneys, respectively. a, g Anterior images. b, h Posterior images. c, i Right anterior oblique images. d, j Left posterior oblique images. e, k Left anterior oblique images. f, i Right posterior oblique images
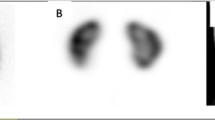
Comparison of planar and single-photon emission computed tomography (SPECT) images in a 3-year-old girl referred for renal scarring 5 months after urinary tract infection with Escherichia coli. Posterior planar image (a) and posterior two-dimensional coronal projection SPECT images at 15 min (b), 10 min (c), 5 min (d) and 2.5 min (e) showed a scar in the upper pole of the right kidney
No significant difference was found between the means of planar and all SPECT series (P > 0.7 for all pairwise comparisons). Bland–Altman plots representing agreement of the split renal function estimation between planar and SPECT series are presented in Fig. 4. No mean difference line was significantly different from zero. In SPECT-2.5 min, limits of agreement increased to 7.5 points, compared to 7.2 points for SPECT-5 min and 7.0 points for SPECT-10 min and SPECT-15 min, respectively. Of 27 patients, 1 (4%) had significantly different split renal function measures in all SPECT series as compared to planar series.
The scores for image quality, resolution and contrast gradually improved with an increase in the duration of SPECT acquisition. Results of these evaluations are detailed in Table 2. The overall image quality score was 1.3 (± 0.6) for the planar data set, 1.6 (± 0.5) for the SPECT-15 min data set, 1.4 (± 0.5) for the SPECT-10 min data set, 1.0 (± 0.5) for the SPECT-5 min data set and 0.6 (± 0.6) for the SPECT-2.5 min data set.
Inter-observer reproducibility between planar and SPECT series results is presented in Table 3. Median Kappa coefficients for inter-observer agreement between planar and SPECT images were greater than 0.83 for all series and all readers except one reader for the SPECT-2.5 min series (median Kappa coefficient = 0.77).
Results of intra-observer reproducibility are presented in Table 4. Median Kappa coefficients for inter-observer agreement between planar and SPECT images were greater than 0.81 for all series and all readers.
Discussion
The present study evaluated the feasibility of DMSA scans with short acquisition times using a SPECT CZT gamma camera and the results suggest that DMSA scans can be reduced to a 5-min SPECT acquisition with minimal impact on images in terms of quality and reproducibility. This finding presents an opportunity to significantly reduce DMSA scan times, which could be especially beneficial for pediatric patients. This significant reduction in scan time could provide greater comfort to children, potentially reducing the likelihood of motion artifacts. Regarding the injected dose, this significant reduction in scan time equates to an 83% decrease in administered activity.
In the past few years, SPECT has begun to replace traditional planar scans; this transition has been accelerated largely by advances in the capabilities of new cameras [10]. However, there have only been a few reports on the potential for reducing scan times and/or injection doses using this new SPECT approach. In agreement with the present study, using previous technology with a conventional Anger gamma camera, Sheehy et al. reported the possibility of a substantial reduction of administered activity and/or scanning time [14]. They compared filter back projection and OSEM-3-D reconstruction methods of DMSA SPECT in terms of image quality and radiopharmaceutical administration and concluded that OSEM-3-D was superior and yielded enhanced image quality allowing a 50% potential reduction of administered activity. Lin et al. recently demonstrated that deep learning-based approaches can generate full-acquisition-time DMSA planar images from short-acquisition-time images, thus reducing the acquisition time in pediatric patients [15]. In addition, SPECT combined with low-dose CT has shown promising results in renal parenchymal assessment [5, 6]. However, additional studies are necessary to evaluate the risk–benefit relationship between the added ionizing radiation and the potential benefit of CT correlation. In this study, we used SPECT-based 2-D reconstructions without the use of low-dose CT for attenuation correction and found that a SPECT CZT gamma camera can produce high-quality DMSA SPECT images from a 5-min SPECT acquisition.
The present findings are subject to certain limitations. This study is a retrospective simulation of reduced-count acquisitions with a small sample size and requires prospective validation. In addition, in order to focus on the influence of reduced scanning time, we chose to use the same reconstruction parameters for all SPECT data sets. Therefore, these parameters may not have been optimal for each series and consequently an alteration of resolution and its consequences cannot be excluded because of an insufficient number of algorithmic iterations. Furthermore, whereas 2-D SPECT is known to be simple and effective for visual analysis of DMSA SPECT images [13], it may not be optimal for quantification and it is of note that a recent study reported by Civan et al. suggests that using 3-D measurement methods could be more accurate for pediatric patients [16]. More generally, a significant portion of the children herein were older than 5 years of age, who are more likely to remain still during the examination and the reproducibility between readers could have been overstated using a binary score for the evaluation of renal size (symmetrical or asymmetrical) and renal contour (preserved or irregular) instead of more quantified measures. Finally, the qualitative assessment was conducted by readers from the same institution and therefore this may differ with readers from different institutions.
Conclusion
Shortening CZT SPECT acquisition time to 5 min is feasible with minimal impact on images in terms of quality and reproducibility.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Do J, Ruddy TD, Wells RG (2021) Reduced acquisition times for measurement of myocardial blood flow with 99mTc-tetrofosmin and solid-state detector SPECT. J Nucl Cardiol 28:2518–2529
Surti S, Viswanath V, Daube-Witherspoon ME et al (2020) Benefit of improved performance with state-of-the art digital PET/CT for lesion detection in oncology. J Nucl Med 61:1684–1690
Dietz M, Kamani CH, Allenbach G et al (2022) Comparison of the prognostic value of impaired stress myocardial blood flow, myocardial flow reserve, and myocardial flow capacity on low-dose Rubidium-82 SiPM PET/CT. J Nucl Cardiol. https://doi.org/10.1007/s12350-022-03155-6
Vali R, Armstrong IS, Bar-Sever Z et al (2022) SNMMI procedure standard/EANM practice guideline on pediatric [99mTc]Tc-DMSA renal cortical scintigraphy: an update. Clin Transl Imaging 10:173–184
Reichkendler MH, Berg RMG, de Nijs R et al (2020) Planar scan vs. SPECT/low-dose CT for estimating split renal function by 99mTc-DMSA scintigraphy in children. Eur J Nucl Med Mol Imaging 47:729–733
Einarsdóttir HS, Berg RMG, Borgwardt L (2020) Interrater reliability of 99mTc-DMSA scintigraphy performed as planar scan vs. SPECT/low dose CT for diagnosing renal scarring in children. Diagnostics (Basel) 10:1101
Mendichovszky I, Solar BT, Smeulders N et al (2017) Nuclear medicine in pediatric nephro-urology: an overview. Semin Nucl Med 47:204–228
Lim R, Bar-Sever Z, Treves ST (2019) Is Availability of 99mTc-DMSA Insufficient to Meet Clinical Needs in the United States? A Survey. J Nucl Med 60:14N-16N
Sarikaya I (2019) 68Ga-PSMA ligand as potential 99mTc-DMSA alternative. J Nucl Med 60:12N-12N
Ito T, Matsusaka Y, Onoguchi M et al (2021) Experimental evaluation of the GE Discovery 870 CZT clinical SPECT system equipped with WEHR and MEHRS collimator. J Appl Clin Med Phys 22:165–177
Ljungberg M, Pretorius PH (2018) SPECT/CT: an update on technological developments and clinical applications. Br J Radiol 91:20160402
Lassmann M, Biassoni L, Monsieurs M et al (2009) The new EANM paediatric dosage card. Eur J Nucl Med Mol Imaging 36:540–541
Cao X, Xu X, Grant FD, Treves ST (2016) Estimation of split renal function with 99mTc-DMSA SPECT: comparison between 3D volumetric assessment and 2D coronal projection imaging. Am J Roentgenol 207:1324–1328
Sheehy N, Tetrault TA, Zurakowski D et al (2009) Pediatric 99mTc-DMSA SPECT performed by using iterative reconstruction with isotropic resolution recovery: improved image quality and reduced radiopharmaceutical activity. Radiology 251:511–516
Lin C, Chang Y-C, Chiu H-Y et al (2021) Reducing scan time of paediatric 99mTc-DMSA SPECT via deep learning. Clin Radiol 76:315.e13-315.e20
Civan C, Simsek DH, Kiran MY et al (2022) Comparison of 2D planar and 3D volumetric methods for estimation of split renal function by 99mTc-DMSA scintigraphy. Phys Medica Eur J Med Phys 95:83–88
Acknowledgements
The authors thank Philip Robinson, PhD, for proofreading the article.
Author information
Authors and Affiliations
Contributions
M.J. conceived, supervised and supported the study. M.D., A.B.S., B.C. and A.F. collated and analyzed the data, performed the statistical analysis and drafted the initial manuscript. B.C., G.P.J., C.M.T., B.G. and M.J. interpreted the images. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its last amendments or comparable ethical standards. The institutional review board approved this retrospective study and waived the requirement for informed patient consent.
Consent for publication
Not applicable.
Conflicts of interest
None
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dietz, M., Jacquet-Francillon, N., Bani Sadr, A. et al. Ultrafast cadmium-zinc-telluride-based renal single-photon emission computed tomography: clinical validation. Pediatr Radiol 53, 1911–1918 (2023). https://doi.org/10.1007/s00247-023-05682-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-023-05682-x