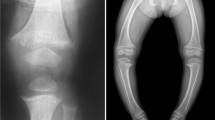
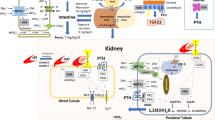
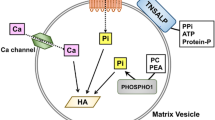
Abstract
Phosphate has extensive physiological roles including energy metabolism, genetic function, signal transduction and membrane integrity. Regarding the skeleton, not only do phosphate and calcium form the mineral component of the skeleton, but phosphate is also essential in regulating function of skeletal cells. Although our understanding of phosphate homeostasis has lagged behind and remains less than that for calcium, considerable advances have been made since the recognition of fibroblast growth factor-23 (FGF23) as a bone-derived phosphaturic hormone that is a major regulator of phosphate homeostasis. In this two-part review of disorders of phosphate homeostasis in children, part 1 covers the basics of mineral ion homeostasis and the roles of phosphate in skeletal biology. Part 1 includes phosphate-related disorders of mineralization for which overall circulating mineral ion homeostasis remains normal. Part 2 covers hypophosphatemic and hyperphosphatemic disorders, emphasizing, but not limited to, those related to increased and decreased FGF23 signaling, respectively.







Similar content being viewed by others
References
Christov M, Jüppner H (2018) Phosphate homeostasis disorders. Best Pract Res Clin Endocrinol Metab 32:685–706
Kinoshita Y, Fukumoto S (2018) X-linked hypophosphatemia and FGF23-related hypophosphatemic diseases: prospect for new treatment. Endocr Rev 39:274–291
Bitzan M, Goodyer PR (2019) Hypophosphatemic rickets. Pediatr Clin N Am 66:179–207
Meyer RA, Meyer MH, Gray RW (1989) Parabiosis suggests a humoral factor is involved in X-linked hypophosphatemia in mice. J Bone Miner Res 4:493–500
Levine BS, Kleeman CR, Felsenfeld AJ (2009) The journey from vitamin D-resistant rickets to the regulation of renal phosphate transport. Clin J Am Soc Nephrol 4:1866–1877
ADHR Consortium (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348
Drezner MK (2001) Tumor-induced osteomalacia. Rev Endocr Metab Disord 2:175–186
Shimada T, Mizutani S, Muto T et al (2001) Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A 98:6500–6505
Jan de Beur SM, Levine MA (2002) Molecular pathogenesis of hypophosphatemic rickets. J Clin Endocrinol Metab 87:2467–2473
Balani S, Perwad F (2019) Fibroblast growth factor 23 and phosphate homeostasis. Curr Opin Nephrol Hypertens 28:465–473
Bar L, Stournaras C, Lang F et al (2019) Regulation of fibroblast growth factor 23 (FGF23) in health and disease. FEBS Lett 593:1879–1900
Vervloet M (2019) Renal and extrarenal effects of fibroblast growth factor 23. Nat Rev Nephrol 15:109–120
Bacchetta J, Bardet C, Prié D (2020) Physiology of FGF23 and overview of genetic diseases associated with renal phosphate wasting. Metabolism 103s:153865
Takashi Y, Fukumoto S (2020) Phosphate-sensing and regulatory mechanism of FGF23 production. J Endocrinol Investig 43:877–883
Figueres L, Beck-Cormier S, Beck L, Marks J (2021) The complexities of organ crosstalk in phosphate homeostasis: time to put phosphate sensing back in the limelight. Int J Mol Sci 22:5701
Tiosano D, Hochberg Z (2009) Hypophosphatemia: the common denominator of all rickets. J Bone Miner Metab 27:392–401
Orriss IR (2020) Extracellular pyrophosphate: the body's "water softener". Bone 134:115243
Orriss IR, Arnett TR, Russell RG (2016) Pyrophosphate: a key inhibitor of mineralisation. Curr Opin Pharmacol 28:57–68
Cui L, Houston DA, Farquharson C, MacRae VE (2016) Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone 87:147–158
Anderson HC, Garimella R, Tague SE (2005) The role of matrix vesicles in growth plate development and biomineralization. Front Biosci 10:822–837
Millán JL (2013) The role of phosphatases in the initiation of skeletal mineralization. Calcif Tissue Int 93:299–306
Bottini M, Mebarek S, Anderson KL et al (2018) Matrix vesicles from chondrocytes and osteoblasts: their biogenesis, properties, functions and biomimetic models. Biochim Biophys Acta Gen Subj 1862:532–546
Kimata M, Michigami T, Tachikawa K et al (2010) Signaling of extracellular inorganic phosphate up-regulates cyclin D1 expression in proliferating chondrocytes via the Na+/pi cotransporter pit-1 and Raf/MEK/ERK pathway. Bone 47:938–947
Michigami T, Ozono K (2019) Roles of phosphate in skeleton. Front Endocrinol 10:180
Millán JL, Whyte MP (2016) Alkaline phosphatase and hypophosphatasia. Calcif Tissue Int 98:398–416
Chande S, Bergwitz C (2018) Role of phosphate sensing in bone and mineral metabolism. Nat Rev Endocrinol 14:637–655
Montaner Ramón A (2020) Risk factors of bone mineral metabolic disorders. Semin Fetal Neonatal Med 25:101068
Chinoy A, Mughal MZ, Padidela R (2019) Metabolic bone disease of prematurity: causes, recognition, prevention, treatment and long-term consequences. Arch Dis Child Fetal Neonatal Ed 104:F560–f566
Schulz EV, Wagner CL (2020) History, epidemiology and prevalence of neonatal bone mineral metabolic disorders. Semin Fetal Neonatal Med 25:101069
Ryan S (1996) Nutritional aspects of metabolic bone disease in the newborn. Arch Dis Child Fetal Neonatal Ed 74:F145–F148
Greer FR (1994) Osteopenia of prematurity. Annu Rev Nutr 14:169–185
Done SL (2012) Fetal and neonatal bone health: update on bone growth and manifestations in health and disease. Pediatr Radiol 42:S158–S176
Viswanathan S, Khasawneh W, McNelis K et al (2014) Metabolic bone disease: a continued challenge in extremely low birth weight infants. JPEN J Parenter Enteral Nutr 38:982–990
Harrison CM, Gibson AT (2013) Osteopenia in preterm infants. Arch Dis Child Fetal Neonatal Ed 98:F272–F275
Mornet E (2018) Hypophosphatasia. Metabolism 82:142–155
Fauvert D, Brun-Heath I, Lia-Baldini AS et al (2009) Mild forms of hypophosphatasia mostly result from dominant negative effect of severe alleles or from compound heterozygosity for severe and moderate alleles. BMC Med Genet 10:51
Lia-Baldini AS, Brun-Heath I, Carrion C et al (2008) A new mechanism of dominance in hypophosphatasia: the mutated protein can disturb the cell localization of the wild-type protein. Hum Genet 123:429–432
Lia-Baldini AS, Muller F, Taillandier A et al (2001) A molecular approach to dominance in hypophosphatasia. Hum Genet 109:99–108
Ornoy A, Adomian GE, Rimoin DL (1985) Histologic and ultrastructural studies on the mineralization process in hypophosphatasia. Am J Med Genet 22:743–758
Shohat M, Rimoin DL, Gruber HE, Lachman RS (1991) Perinatal lethal hypophosphatasia; [sic] clinical, radiologic and morphologic findings. Pediatr Radiol 21:421–427
Zhang Z, Nam HK, Crouch S, Hatch NE (2021) Tissue nonspecific alkaline phosphatase function in bone and muscle progenitor cells: control of mitochondrial respiration and ATP production. Int J Mol Sci 22:1140
Khan AA, Josse R, Kannu P et al (2019) Hypophosphatasia: Canadian update on diagnosis and management. Osteoporos Int 30:1713–1722
Whyte MP, Zhang F, Wenkert D et al (2015) Hypophosphatasia: validation and expansion of the clinical nosology for children from 25 years experience with 173 pediatric patients. Bone 75:229–239
Linglart A, Biosse-Duplan M (2016) Hypophosphatasia. Curr Osteoporos Rep 14:95–105
Villa-Suárez JM, García-Fontana C, Andújar-Vera F et al (2021) Hypophosphatasia: a unique disorder of bone mineralization. Int J Mol Sci 22:4303
Kozlowski K, Sutcliffe J, Barylak A et al (1976) Hypophosphatasia. Review of 24 cases. Pediatr Radiol 5:103–117
Shore RM (2008) Metabolic bone disease. In: Slovis TL (ed) Caffey's pediatric diagnostic imaging, 11th edn. Elsevier, Philadelphia, pp 2726–2752
Wenkert D, McAlister WH, Coburn SP et al (2011) Hypophosphatasia: nonlethal disease despite skeletal presentation in utero (17 new cases and literature review). J Bone Miner Res 26:2389–2398
Whyte MP, Greenberg CR, Salman NJ et al (2012) Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med 366:904–913
Whyte MP (2017) Hypophosphatasia: enzyme replacement therapy brings new opportunities and new challenges. J Bone Miner Res 32:667–675
Rush ET (2018) Childhood hypophosphatasia: to treat or not to treat. Orphanet J Rare Dis 13:116
Boyce AM, Gafni RI, Ferreira CR (2020) Generalized arterial calcification of infancy: new insights, controversies, and approach to management. Curr Osteoporos Rep 18:232–241
Nitschke Y, Yan Y, Buers I et al (2018) ENPP1-fc prevents neointima formation in generalized arterial calcification of infancy through the generation of AMP. Exp Mol Med 50:1–12
Ferreira CR, Hackbarth ME, Ziegler SG et al (2021) Prospective phenotyping of long-term survivors of generalized arterial calcification of infancy (GACI). Genet Med 23:396–407
Chong CR, Hutchins GM (2008) Idiopathic infantile arterial calcification: the spectrum of clinical presentations. Pediatr Dev Pathol 11:405–415
Ferreira CR, Kintzinger K, Hackbarth ME et al (2021) Ectopic calcification and hypophosphatemic rickets: natural history of ENPP1 and ABCC6 deficiencies. J Bone Miner Res 36:2193–2202
Hajjawi MO, MacRae VE, Huesa C et al (2014) Mineralisation of collagen rich soft tissues and osteocyte lacunae in Enpp1(−/−) mice. Bone 69:139–147
Acknowledgments
I would like to thank Aaron L. Friedman, MD, for his helpful advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shore, R.M. Disorders of phosphate homeostasis in children, part 1: primer on mineral ion homeostasis and the roles of phosphate in skeletal biology. Pediatr Radiol 52, 2278–2289 (2022). https://doi.org/10.1007/s00247-022-05374-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-022-05374-y