Abstract
We previously reported short-term outcomes for stenting of aortic coarctation (CoA) (native or re-coarctation) with newer generation low-profile stents (Valeo, Formula, and Begraft stents) in children under 30 kg. We present here the medium-term outcomes of this procedure. Retrospective review of patients weighing under 30 kg who had percutaneous stent treatments for coarctation between 2012 and 2021 was performed. Clinical and procedural data were collected; 19 patients were included. The median age at the time of procedure was 5.1 [4.1–6.4] years and median weight 21.0 [17.3–22.3] kg. One patient had a history of re-coarctation. Thirteen (68%) patients were on anti-hypertensives pre-procedure. Different types of stents were used (14 Valeo™, 4 Formula® 535, 1 BeGraft), which can all be dilated to 18 mm or larger. One patient required a 9 F sheath, all others required a 7 F sheath. The narrowest diameter in the aorta increased from a median of 3.5 [3.0–4.5] to 9.4 [8.9–9.8] mm, p < 0.001; there was a reduction in the median pressure gradient across the coarctation from 35.0 [30.0–43.0] to 5.0 [0–10.0] mmHg, p < 0.001. There were no intra-procedural complications. Follow-up was for a median of 56.0 [13.0–65.0] months. Five (26%) of patients underwent re-intervention after a median time frame of 40.0 [39.5–52.0] months; four had balloon dilation, one had repeat stent implantation. Five (26%) patients were on anti-hypertensive agent(s) post-intervention. Our single centre experience demonstrates that percutaneous stenting for coarctation of aorta in children under 30 kg, with low-profile stents, had no significant complications during the median follow-up time of 56 months. This study demonstrated that the procedure is safe and effective for short and medium-term therapy in this group of patients with a 26% re-intervention rate. A quarter of patients remained on anti-hypertensive medication post stenting, emphasizing the importance of long-term follow-up.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Aortic coarctation (CoA) has an overall incidence of 5/10,000 live births and, if left untreated, can lead to significant morbidity and mortality [1, 2]. Compared to balloon angioplasty alone, stent implantation for CoA provides superior dilation and avoids acute re-modelling of the aortic wall [3,4,5]. Stent delivery is limited by femoral artery size in small children. The COAST trial demonstrated the feasibility and safety of coarctation stenting in a large prospective multicentre study, however, all patients in this study were over 30 kg [6]. We previously reported our short-term outcomes of CoA treatment using Valeo™ (BD, NJ, USA) stents in children weighing under 30 kg, which can be dilated to adult size (18 mm) [7]. We now report the medium-term outcomes of CoA stenting in our centre. We aimed to report the need for ongoing anti-hypertensive therapy, re-intervention and peri-procedural, short and medium-term complications related to the procedure.
Methods
As described previously, all patients weighing under 30 kg with a diagnosis of coarctation of the aorta, who underwent stent implantation in our centre, were identified from the departmental cardiology database (HeartSuite™) [7]. Patient demographics and clinical data were collected from the medical notes and HeartSuite™. Angiographic and haemodynamic data, including the diameter of the coarctation and pressure gradients before and after stent implantation were collated from catheterisation reports and angiograms and complications assessed using the clinical notes and electronic records. Vascular complications included vascular occlusion, arteriovenous fistulae or pseudo-aneurysms, identified on ultrasound if there had been clinical suspicion of vascular injury. Cerebral complications included cerebral infarction or bleeding, based on clinical suspicion or confirmed by brain MRI or computer tomography (CT). We also assessed for evidence of aortic dissection, aneurysm or pseudoaneurysm formation, stent fracture, deformation or migration or neo-intimal proliferation on CT and angiography for those requiring re-intervention.
Data are expressed as medians with interquartile ranges [IQR] and percentages as appropriate. Comparisons before and after stent implantation were performed using the paired Student’s t-test and a p value <0.05 was considered significant. All statistical analyses were performed using IBM® SPSS® Statistics Version 27 (IBM, Armonk, New York).
Stent Descriptions
The Valeo stent (BD, NJ, USA) is a bare metal stent that has a triple helix design laser cut from a 316-L stainless steel tube. The stent is pre-mounted on a low-profile nylon balloon accepting a 0.035-in. guide wire and accommodating inflation pressures up to 14 atm. The stent is available in diameters between 6 and 10 mm and lengths between 18 and 56 mm. The required sheath size is 6 F for stents mounted on 6–8 mm balloons and 7 F for the larger stents mounted on 9–10 mm balloons. The open cell architecture of the stent allows it to be post dilated with minimal shortening [8, 9]. The 6–8 mm Valeo stents can be maximally dilated to 13 mm before fracturing. In our case series, only the 9 and 10 mm Valeo stents are used to take advantage of its maximal expandability to 20 mm.
The Formula 535 Vascular Stent (Cook, IN, USA) is a bare metal stent that has a slotted tube configuration made of 316L stainless steel. The stent is pre-mounted on a balloon catheter and deployed over a 0.035 inch guidewire with nominal and burst pressures of 8 and 10 atm, respectively. The stent is available in diameters between 4 and 10 mm and lengths between 12 and 60 mm. The sheath size is 7 F for 9–10 mm diameters stents, 6 F for 5–8 mm diameter stents, and 5 F for 4 mm diameter stents. The 10 and 9 mm diameter stent is dilatable up to maximal 20 mm without significant shortening or loss of stent integrity [10].
The BeGraft stent (Bentley, Hechingen, Germany) is covered with a 200 µm expanded polytetrafluoroethylene (ePTFE) tubing. It is made of Cobalt–Chromium with an open cell design and pre-mounted on a balloon catheter, which goes over a 0.035 inch guidewire. The stent is available in lengths of 19, 29, 39, 49 and 59 mm for diameter sizes of 12 and 14 mm. It is also available in lengths of 19, 29, 38, 48 and 58 mm for diameter sizes of 16 and 18 mm. Finally, it is available in lengths of 27, 37 and 48 mm for diameter sizes of 20, 22, and 24 mm. The 12 and 14 mm diameter stents can be dilated up to maximum diameters of 20 mm, 16 and 18 mm diameter stents to 24 mm, and 20, 22, and 24 mm diameter stents to 30 mm, The required sheath sizes are 9 F (12 mm diameter stent), 11 F (14 and 16 mm), and 14 F (18 mm and above). The nominal and burst pressure for the 12 mm diameter stent is 7 and 10 atm, respectively. The 14 mm diameter stent is 7 and 10 atm, and 16 mm diameter stent is 6 and 9 atm, respectively [11, 12].
Procedure
All stent implantations were performed under general anaesthesia. Valeo™ and Formula® 535 stents were implanted as previously described [7] (Fig. 1). For BeGraft stents (Fig. 2), a 9 F Mullins design 63 cm sheath (Cook, IN, USA) was used to deliver the stent.
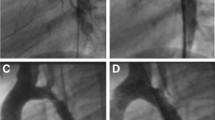
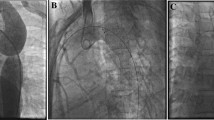
Aortograms and CT of case 17 using Formula 535 stent. A Lateral aortogram demonstrating the descending thoracic CoA. B Lateral aortogram demonstrating a well-expanded 9 mm × 30 mm stent in position with vessel calibre matching. C CT performed 3 months post stent demonstrating no aortic wall complications or stent fractures
Aortograms and CT of a case using a BeGraft stent. A Lateral aortogram shows the left subclavian artery arising posteriorly immediately followed by a severe CoA. B Lateral aortogram showing a well-expanded 12 mm × 19 mm stent in position with matching vessel calibre. C CT performed 3 months after stent implanting demonstrating no aortic wall complications and preserved stent integrity. Although the stent edge partially covered the origin of the left subclavian artery the patient remained asymptomatic
Results
Nineteen patients were included in this study (Table 1). Seven weighed under 20 kg at intervention; all others weighed 20–30 kg. Three types of stents were implanted between 2012 and 2021; 14 Valeo™, 4 Formula® 535, and 1 BeGraft stent. The median age at the time of procedure was 5.1 [4.1–6.4] years and median weight 21.0 kg [17.3–22.3]. One patient had a history of re-coarctation. Thirteen (68%) patients were on anti-hypertensive medications pre-procedure. 7 F sheaths were used in all but one patient, who required a 9 F sheath for a BeGraft stent (Table 2). All patients received follow-up computer tomography (CT) at 3 months. There was no evidence of aortic dissection, aneurysm or pseudoaneurysm formation, stent fracture, deformation or migration or neo-intimal proliferation on CT in any patients. Patients requiring re-intervention showed no evidence of these complications on re-angiogram and there was no stent foreshortening on stent re-dilation (Table 2).
There was improvement in median coarctation diameter from 3.5 [3.0–4.5] to 9.4 [8.9–9.8] mm, p < 0.001; and a reduction in the median pressure gradient across the coarctation from 35.0 [30.0–43.0] to 5.0 [0–10.0] mmHg, p < 0.001. There were no short or medium-term complications related to the procedure including the pre-specified cerebral and vascular complications (Table 1).
Follow-up was for a median of 56.0 [13.0–65.0] months. Five (26%) of patients underwent re-intervention; four patients had balloon dilation of the stent, one of which has required who procedures and one had balloon angioplasty followed by immediate stent implant due to a high residual gradient. within a median time frame of 45 [40–53] months. Five (26%) patients were on anti-hypertensive agent(s) post-intervention. Again, none of the aforementioned vascular complications or cerebral complications specified in the methods were present on follow-up.
Discussion
In the last two decades, stent implantation has emerged as an alternative to surgery in adults and adolescents with CoA and has had good outcomes [3, 5, 13,14,15]. In small children, stenting is limited by the small number of available stents that can be dilated to adult size, to keep up with somatic growth and the large size of the delivery sheaths, which increase the risk of vascular complications [5, 16,17,18]. These challenges are being overcome with newer generation stents [7, 19, 20].
Other larger studies have assessed the feasibility of coarctation stenting including one large multicentre study (the COAST) trial. However, the smallest patient in the study was 35 kg [6].
Boe and colleagues demonstrated the feasibility of coarctation stenting in small patients under 20 kg [20]. Their study differs to our due to their use of Palmaz stents, which, unlike the more modern stents used in the present study, are not pre-mounted and require manual mounting and crimping, increasing the risk of error and complications such as “hour-glassing”. In addition, a 9 F or larger sheath was used in nearly 20% of patients in their cohort, where as a 9 F sheath was used in only one case in ours. Whilst Boe et al. were able to demonstrate the feasibility of coarctation stenting in small patients; their complication rate was much higher at around 18%. The present study suggests the superiority of newer generation stents for small patients, including those weighing under 20 kg.
We previously described short term follow-up of coarctation stenting in children under 30 kg using a low-profile balloon expandable [7]. The three stent types that were described could all be introduced via arterial sheaths of 7–9 F in size, which are suitable for small children. In our study, only one patient out of 19 had transiently reduced femoral pulse, which resolved after administration of tissue plasminogen activator. Follow-up vascular ultrasound at 3 weeks showed no complications.
In addition, these low-profile stents can be re-dilated and have the potential to reach adult size. As described previously, the 9- and 10-mm diameter Valeo stent can be dilated up to 20 mm with no loss of stent integrity or length [9]. The BeGraft 12 mm diameter stent up to 20 mm with no significant compromise on the length or integrity of the stent [11]. Bench testing of the Formula 535 stent demonstrated that the 10 mm stents can be dilated up to 20 mm [10]. Use of a short balloon (approximately the same length of the stent) was recommended to prevent “dog-boning” of the stent i.e. preferential expansion of the lateral aspects compared to the middle. They noted that with over-dilation, the Formula stents maintained their length with no shortening, allowing precision placement and minimal protrusion. More recently, we have moved to using these Formula stents where possible and did so in four of the six most recent patients published here due to its’ favourable dilation potential without foreshortening [10].
Indications for stent re-dilation have been varied (Table 2) and are based on clinical and imaging data and a general need to keep with somatic growth. Despite the concerns of the somatic growth spurt in small children and need for re-intervention, in our study, only 5 (26%) of our patient needed re-intervention. Hence, these low-profile stents appear to be safe and effective option to treat CoA in small children.
Thus far, none of the implanted stents have required over-dilation to sizes approximating the maximum achieved dilation on bench testing and no stent fractures have been observed in this cohort. Data from Crystal et al. and Danon et al. demonstrated the feasibility and reliability of intentional stent fracture without “napkin-ring” formation to allow for re-stenting with somatic growth with Formula and Valeo stents [21, 22]. Future publication of longer-term outcomes from our cohort requiring over-dilation will be highly informative.
Of note, the Formula 535 stent is, to date, not FDA approved for use in the United States. The approved Formula 418 stent, which is mounted over a smaller wire, offers an alternative. However, unlike the 14-crown Formula 535 configuration, the 12-crown configuration Formula 418 stent is only available up to 8 mm and can only be post dilated to 14–15 mm before losing it’s radial strength or fracturing [22].
At follow-up, five (26%) of our patients remained on anti-hypertensives over a median follow-up of 56 months despite successful gradient reduction post-intervention and imaging evidence of stent re-stenoses on CT. This is consistent with previous reports that showed 37–57% of patients with CoA having residual hypertension or remain on anti-hypertensives despite correction of anatomical substrate [15, 23, 24]. The pathophysiology is not well understood, however there are reports that suggest the hypertension in CoA is not only contributed by the mechanical obstruction but also several other factors such as arch hypoplasia, abnormal aortic compliance, vascular stiffness and abnormal baroreceptor function resulting in diffuse arteriopathy [25, 26]. Hence, lifelong surveillance is imperative following intervention for those with CoA.
Similar to our previous report [7], this study is limited by its retrospective single-centre design and small patient sample.
Conclusions
Use of small profile balloon expandable stents is a feasible and effective long-term treatment option in children under 30 kg with CoA, with very low risk of complications. Repeated balloon dilations to match somatic growth, is to be expected but did not have to be repeated frequently in our series with only 5 out of 19 patients requiring re-intervention during the period of follow-up.
References
Campbell M (1970) Natural history of coarctation of the aorta. Br Heart J 32(5):633–640
Hoffman JI, Kaplan S (2002) The incidence of congenital heart disease. J Am Coll Cardiol 39(12):1890–1900
Forbes TJ, Kim DW, Du W, Turner DR, Holzer R, Amin Z et al (2011) Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: an observational study by the CCISC (Congenital Cardiovascular Interventional Study Consortium). J Am Coll Cardiol 58(25):2664–2674
Magee AG, Brzezinska-Rajszys G, Qureshi SA, Rosenthal E, Zubrzycka M, Ksiazyk J et al (1999) Stent implantation for aortic coarctation and recoarctation. Heart 82(5):600–606
Chessa M, Carrozza M, Butera G, Piazza L, Negura DG, Bussadori C et al (2005) Results and mid-long-term follow-up of stent implantation for native and recurrent coarctation of the aorta. Eur Heart J 26(24):2728–2732
Meadows J, Minahan M, McElhinney DB, McEnaney K, Ringel R, COAST Investigators* (2015) Intermediate outcomes in the prospective, multicenter coarctation of the aorta stent trial (COAST). Circulation 131(19):1656–64
Kang SL, Tometzki A, Taliotis D, Martin R (2017) Stent therapy for aortic coarctation in children <30 kg: use of the low profile Valeo stent. Pediatr Cardiol 38(7):1441–1449
Stern HJ, Baird CW (2009) A premounted stent that can be implanted in infants and re-dilated to 20 mm: introducing the Edwards Valeo Lifestent. Catheter Cardiovasc Interv 74(6):905–912
Travelli FC, Sullivan PM, Takao C, Ing FF (2016) The Valeo stent: a pre-mounted, open-cell, large stent for use in small children with CHD. Cardiol Young 26(6):1187–1193
Quandt D, Ramchandani B, Bhole V, Penford G, Mehta C, Dhillon R et al (2015) Initial experience with the cook formula balloon expandable stent in congenital heart disease. Catheter Cardiovasc Interv 85(2):259–266
Promphan W, Han Siang K, Prachasilchai P, Jarutach J, Makonkawkeyoon K, Siwaprapakorn W et al (2020) Feasibility and early outcomes of aortic coarctation treatments with BeGraft Aortic stent. Catheter Cardiovasc Interv 96(3):E310–E316
Al Balushi A, Pascall E, Jones MI, Qureshi S, Butera G (2021) Initial experience with a novel ePTFE-covered balloon expandable stent in patients with near-atretic or severe aortic coarctation and small femoral arterial access. Cardiol Young 31(2):224–228
Carr JA (2006) The results of catheter-based therapy compared with surgical repair of adult aortic coarctation. J Am Coll Cardiol 47(6):1101–1107
Butera G, Manica JL, Marini D, Piazza L, Chessa M, Filho RI et al (2014) From bare to covered: 15-year single center experience and follow-up in trans-catheter stent implantation for aortic coarctation. Catheter Cardiovasc Interv 83(6):953–963
Holzer RJ, Gauvreau K, McEnaney K, Watanabe H, Ringel R (2021) Long-term outcomes of the coarctation of the aorta stent trials. Circ Cardiovasc Interv 14(6):e010308
Rosenthal E (2005) Coarctation of the aorta from fetus to adult: curable condition or life long disease process? Heart 91(11):1495–1502
Ewert P, Schubert S, Peters B, Abdul-Khaliq H, Nagdyman N, Lange PE (2005) The CP stent—short, long, covered—for the treatment of aortic coarctation, stenosis of pulmonary arteries and caval veins, and Fontan anastomosis in children and adults: an evaluation of 60 stents in 53 patients. Heart 91(7):948–953
Golden AB, Hellenbrand WE (2007) Coarctation of the aorta: stenting in children and adults. Catheter Cardiovasc Interv 69(2):289–299
Gendera K, Ewert P, Tanase D, Georgiev S, Genz T, Bambul Heck P et al (2018) Balloon-expandable stents for recoarctation of the aorta in small children. Two centre experience. Int J Cardiol 263:34–39
Boe BA, Armstrong AK, Janse SA, Loccoh EC, Stockmaster K, Holzer RJ et al (2021) Percutaneous implantation of adult sized stents for coarctation of the aorta in children </=20 kg: a 12-year experience. Circ Cardiovasc Interv 14(2):e009399
Crystal MA, Morgan GJ, Danon S, Gray RG, Gruenstein DH, Gordon BM et al (2018) Serial versus direct dilation of small diameter stents results in a more predictable and complete intentional transcatheter stent fracture: a PICES Bench Testing Study. Pediatr Cardiol 39(1):120–128
Danon S, Gray RG, Crystal MA, Morgan G, Gruenstein DH, Goldstein BH et al (2016) Expansion characteristics of stents used in congenital heart disease: serial dilation offers improved expansion potential compared to direct dilation: results from a Pediatric Interventional Cardiology Early Career Society (PICES) investigation. Congenit Heart Dis 11(6):741–750
Hager A, Kanz S, Kaemmerer H, Schreiber C, Hess J (2007) Coarctation Long-term Assessment (COALA): significance of arterial hypertension in a cohort of 404 patients up to 27 years after surgical repair of isolated coarctation of the aorta, even in the absence of restenosis and prosthetic material. J Thorac Cardiovasc Surg 134(3):738–745
Holzer R, Qureshi S, Ghasemi A, Vincent J, Sievert H, Gruenstein D et al (2010) Stenting of aortic coarctation: acute, intermediate, and long-term results of a prospective multi-institutional registry—Congenital Cardiovascular Interventional Study Consortium (CCISC). Catheter Cardiovasc Interv 76(4):553–563
de Divitiis M, Pilla C, Kattenhorn M, Zadinello M, Donald A, Leeson P et al (2001) Vascular dysfunction after repair of coarctation of the aorta: impact of early surgery. Circulation 104(12 Suppl 1):I165–I170
Vogt M, Kuhn A, Baumgartner D, Baumgartner C, Busch R, Kostolny M et al (2005) Impaired elastic properties of the ascending aorta in newborns before and early after successful coarctation repair: proof of a systemic vascular disease of the prestenotic arteries? Circulation 111(24):3269–3273
Author information
Authors and Affiliations
Contributions
JJCG and WCK collected data, prepared figures, tables, and critically reviewed the manuscript. FGB collected data and gave critical review of the manuscript. WCK, JJCG, and DT wrote the manuscript text. AT, MC, and AP collected data and provided critical review of the data and the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors did not receive support from any organization for the submitted work. The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gibb, J.J.C., Kim, W.C., Barlatay, F.G. et al. Medium-Term Outcomes of Stent Therapy for Aortic Coarctation in Children Under 30 kg with New Generation Low-Profile Stents: A Follow-Up Study of a Single Centre Experience. Pediatr Cardiol 45, 544–551 (2024). https://doi.org/10.1007/s00246-023-03402-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-023-03402-8