Abstract
The Single Ventricle Reconstruction (SVR) Trial was a randomized prospective trial designed to determine survival advantage of the modified Blalock-Taussig-Thomas shunt (BTTS) vs the right ventricle to pulmonary artery conduit (RVPAS) for patients with hypoplastic left heart syndrome. The primary aim of the long-term follow-up (SVRIII) was to determine the impact of shunt type on RV function. In this work, we describe the use of CMR in a large cohort follow up from the SVR Trial as a focused study of single ventricle function. The SVRIII protocol included short axis steady-state free precession imaging to assess single ventricle systolic function and flow quantification. There were 313 eligible SVRIII participants and 237 enrolled, ages ranging from 10 to 12.5 years. 177/237 (75%) participants underwent CMR. The most common reasons for not undergoing CMR exam were requirement for anesthesia (n = 14) or ICD/pacemaker (n = 11). A total of 168/177 (94%) CMR studies were diagnostic for RVEF. Median exam time was 54 [IQR 40–74] minutes, cine function exam time 20 [IQR 14–27] minutes, and flow quantification time 18 [IQR 12–25] minutes. There were 69/177 (39%) studies noted to have intra-thoracic artifacts, most common being susceptibility artifact from intra-thoracic metal. Not all artifacts resulted in non-diagnostic exams. These data describe the use and limitations of CMR for the assessment of cardiac function in a prospective trial setting in a grade-school-aged pediatric population with congenital heart disease. Many of the limitations are expected to decrease with the continued advancement of CMR technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Single Ventricle Reconstruction (SVR) Trial was the first of its kind—a prospective randomized trial in congenital heart surgery designed to evaluate the effect of systemic to pulmonary shunt type on short-term survival [1]. The original aim of the SVR Trial was to compare transplantation-free survival in patients with single right ventricle (RV) anatomy, randomizing the patients into two groups, those who received a modified Blalock Taussig Thomas shunt (BTTS) and those who received a right ventricle to pulmonary artery conduit (RVPAS). In the original SVR Trial, early survival benefit was identified for the RVPAS group, but by one year of age, there was no difference in survival between the two shunt types [1]. The SVR cohort has now been studied in follow up work including SVR II [2], aimed at evaluating transplant-free survival following the Glenn and Fontan, and SVR III, the current long-term follow up evaluation of transplant-free survival and single ventricle function into adolescence. The current trial includes the opportunity to evaluate cardiac function in the single right ventricle.
Cardiac magnetic resonance imaging (CMR) is the gold standard for the assessment of right and left ventricular function and myocardial viability in congenital heart disease due to its superior blood-tissue contrast, 3-dimensional imaging, and quantification of blood flow, valve regurgitation, and chamber volumes [3, 4]. Despite the integration of structural and functional imaging into a comprehensive cardiac examination, CMR is viewed as an adjunctive imaging tool and its use in the pediatric population for prospective research has not been well described. A historic limitation to use of CMR prospectively for clinical trials has been the need for sedation or anesthesia in younger children. Clinical guidelines for CMR imaging are published, and many centers have performed CMR without sedation successfully in an increasingly younger population. Newer imaging techniques tailored to decrease scan times and reduce motion artifact, in combination with new child centered imaging environments that include CMR compatible video goggles and child life specialists, improve success rates for non-sedated pediatric CMR studies [5, 6]. The ability to perform CMR without sedation is an important consideration when planning a prospective study and aiming to minimize potential risk.
Given the inherent advantages of CMR for assessment of RV function, the SVR III trial was designed with right ventricular ejection fraction (RVEF) by CMR as the primary outcome measure. Taking into account the improvements over time in the use of CMR in younger children, this specific protocol was designed to overcome concerns regarding the feasibility of using CMR data as the prospective outcome of a clinical trial in 10–12-year-olds with hypoplastic left heart syndrome who have undergone Fontan operations. The objective of this report is to provide a detailed, modern assessment of the use and limitations of CMR in this prospective research setting.
Materials and Methods
This study is a descriptive design to evaluate the use of CMR in the SVR long-term follow-up cohort using standard CMR methods [7]. The design and results of the original SVR study, supported by the Pediatric Heart Network (PHN), and the first follow up study, SVR II, have been previously published [1, 2]. There were originally 555 participants enrolled, with 275 in the BTTS group, 274 in the RVPAS group, and 6 exclusions. Transplant free survivors of the original SVR trial were eligible for enrollment in SVR III. The primary outcome measure was RVEF as measured by CMR. The Institutional Review Board of each participating center approved this study. The parents and participants provided informed consent and assent prior to participation.
As a part of the SVR III cohort study, participants were asked to undergo a non-contrast, non-sedated CMR exam to assess single ventricle function, blood flow in the major venous and arterial vessels including the superior vena cava (SVC), inferior vena cava (IVC)/Fontan conduit, right pulmonary artery (RPA), left pulmonary artery (LPA), neo-ascending aorta, descending aorta, pulmonary veins and tricuspid inflow. Exclusion criteria from CMR exam were the presence of an implantable device, including pacemakers and implantable defibrillators, or the need for anesthesia or sedation. Patients would still be enrolled in the SVR III study regardless of CMR study participation. If the participants underwent a clinically indicated CMR exam requiring contrast or sedation for clinical diagnostic purposes, and it included SVR III protocol components, it was used for analysis. The trial design, methodology, and complete SVR III protocol was published elsewhere. [8]
MRI protocol: The CMR protocol was designed by a small committee of representatives from participating PHN sites. All studies were performed on 1.5 T scanners (Phillips, General Electric, or Siemens). Participants were studied while freely breathing except as otherwise indicated. Scans were acquired with retrospective ECG gating, maximum parallel imaging acceleration factor of 2, and 3 signal averages. The use of a spoiled gradient recalled echo was encouraged in the setting of prominent susceptibility artifact. The CMR protocol was performed in a stepwise fashion, prioritizing SSFP cine data for measurement of RVEF, and included:
-
1.
ECG gated 3 plane localizers
-
2.
ECG gated balanced steady-state free precession (SSFP) cines: RV 2-chamber (vertical long axis), RV “4-chamber” (horizontal long axis), short axis stack through entire RV, and 3-chamber (outflow tract). Slice thickness 5–7 mm, pixel dimensions < 1.5 × 1.5 mm^2, TR < 4 ms, and TE ~ 2 ms.
-
3.
Non-contrast MRA: 3D respiratory navigator and ECG gated SSFP acquisition from the mid-liver to top of the aortic arch with acquired resolution 1.5 mm^3. The trigger delay was optimized for the heart rate and the shot duration was maintained < 130 ms.
-
4.
Phase contrast velocity acquisitions: SVC, IVC/Fontan, RPA, LPA, Neo ascending aorta, pulmonary veins, and tricuspid valve inflow
If CMR were performed as a clinically indicated exam and intravenous gadolinium contrast agent were used, additional data could include:
-
1.
Contrast enhanced magnetic resonance angiography (MRA) from the liver to top of the aortic arch during an inspiratory breath hold.
-
2.
Gradient echo inversion recovery late gadolinium enhancement (LGE) in the short axis stack, 2-chamber (VLA), 4-chamber (HLA), and 3-chamber (outflow tract) planes. LGE images were acquired with an expiratory breath hold if possible.
-
3.
Flow analysis in other vessels not included in the standard research protocol above could also be included.
The anonymized CMR studies were transferred to the core laboratory via a commercial medical image cloud service (Ambra Health, New York). Before quantitative analysis study image quality was graded by the core laboratory on a 0–4 scale.
-
0-uninterpretable
-
1-poor: major artifacts compromising all data
-
2-fair: major artifacts compromising some data
-
3-good: minor artifacts without data compromise
-
4-excellent: no or minimal artifacts.
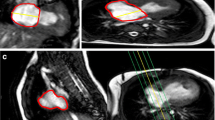
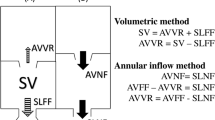
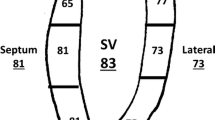
Study artifacts included missing slices, incomplete anatomical coverage, velocity aliasing, susceptibility effects due to intravascular devices and coils, patient motion, and ineffective cardiac gating. Images were determined to be adequate for analysis by two reviewers, and images had to be of a quality that allowed for endocardial and epicardial border identification over the entire ventricular mass and volume. Images were analyzed with CVI42 (Circle Cardiovascular Imaging, Calgary, Alberta). Image analysis included standard planimetry for ventricular volumes and ejection fraction and phase contrast velocity measurement. RV ejection fraction was the study primary outcome variable. If available, vessel diameter measurements were made on the non-contrast 3D SSFP MRA. DICOM header information was used to calculate the total scan, short axis function, and phase contrast velocity imaging times. Image time could not be calculated for some datasets due to the DICOM header information being removed during anonymization.
Results
At the start of SVRIII, 313 patients were eligible for enrollment, and 237 patients enrolled with a median age of 10.76 years [IQR 10.28, 11.35] at the time of consent, Fig. 1. A total of 177/237 (74.7%) CMR studies were performed in participants with a median age of 10.90 [IQR 10.38, 11.45] at the time of CMR exam. Sixty patients did not have a CMR study, and the most common reason for not obtaining a CMR was the presence of a medical device that was not MRI safe (n = 19, 10 pacemaker/ICD). Other common reasons for not having CMR performed were the need for anesthesia (n = 14), refusal to undergo CMR, loss to follow up, claustrophobia, not able to complete prior to close of study, COVID-19 restrictions, and heart transplantation prior to obtaining CMR, Table 1.
The 177 CMR studies were performed at sites with scanners from three vendors: GE (18 exams), Philips (87 exams), and Siemens (72 exams). 135/177 (76%) studies were performed for the purpose of research only, without sedation or contrast. There were 42/177 (24%) studies performed for a clinical indication. There were 29/177 (16%) total studies performed using a gadolinium contrast agent. There were 10/177 (6%) total studies performed under generalized endotracheal tube anesthesia (n = 8) or iv sedation without intubation (n = 2). Therefore, 24 patients (10% of total enrollment, n = 237) either had a CMR under sedation/anesthesia or did not undergo CMR for research purposes due to the need for sedation/anesthesia. Most studies (98) were graded as “good” image quality and only 2 were graded as “uninterpretable” with another 7 that were graded as poor and could not measure the primary outcome, RVEF. The median image quality score was 3 [IQR 2, 3], Fig. 2. There were 168 studies (95% of those performed) of adequate quality to measure the RV ejection fraction. Table 2 lists the type of artifacts present.
The median total exam time was 54 min [IQR 40, 74]. There was high variability in the total exam time—minimum 8 min and maximum 154 min. The variability was secondary to sites adding LGE imaging or other pulse sequences to the base required exam, which is expected with the addition of imaging and contrast injection. Short exam times were from aborted studies or limited studies due to artifact. The median time to acquire adequate SSFP cine images for the primary outcome measure was 20 min [IQR 14, 27]. Similarly, the time for phase contrast image acquisition was 18 min [IQR 12, 25].
Discussion
In the long-term follow-up of the Single Ventricle Reconstruction Trial [1] cohort, the primary outcome measure was RVEF as measured by CMR. The data presented currently assess the feasibility of obtaining this measure in the follow-up SVR population of 10 to 12.5-year-old patients with hypoplastic left heart syndrome status post Fontan surgery. CMR use as a research tool has been well described. However, few studies have provided a thorough review of the success and failure of obtaining CMR in a pediatric cohort, which is important for planning and powering prospective research. Furthermore, in a large cross-sectional study examining Fontan patients between 6 and 18 years of age with pre-determined exclusion criteria, only 159 patients (29%) underwent cardiac MRI out of 546 patients enrolled [3]. These data show CMR was successfully obtained in 75% of enrolled patients and single RV function was successfully obtained in 95% of those who underwent CMR. Exam times were short, and as a proportion of total enrollment, the need for anesthesia was infrequent. This study establishes baseline metrics for CMR as a safe and feasible functional outcome measure in pediatric clinical trials involving complex congenital heart disease.
Earlier data from the SVR Trial suggested that RV function as measured by echocardiography was impaired prior to Fontan surgery in patients who were palliated with a RVPAS versus those who received a mBTTS [9]. This raised the question of whether this surgical strategy, which includes right ventriculotomy in the neonatal period, could lead to long-term RV dysfunction and potentially worse Fontan outcomes. However, ventricular function in complex congenital heart disease, including the single RV, is best assessed by CMR due to its geometric independence for assessment of volumes and ejection fraction [3]. There is a paucity of published data utilizing CMR in a trial setting for measurement of ventricular function in elementary school-aged children with congenital heart disease, and it has been understood that school-aged children, particularly those with developmental impairments which are common for children with congenital heart disease, may struggle to cooperate with CMR examination due to the length of the study and the MRI environment [10,11,12,13]. Neurocognitive deficits are common in patients with single ventricle congenital heart disease with up to 30 to 40% of patients diagnosed with learning disabilities or having an individual educational plan [14, 15]. This is compared to only 5.9% of the enrolled participants who required anesthesia, suggesting that the high incidence of neurocognitive delays should not preclude the use of CMR in pediatric single ventricle research.
While 75% of the patients in this study were able to undergo CMR and nearly all of those had interpretable RV function data, 25% of the patients were unable to undergo CMR. The two most common reasons were MRI-unsafe implanted devices and need for sedation or anesthesia. Over time, the number of patients excluded from CMR studies for both of these reasons can be decreased with advances in device technology, CMR technique and the MRI environment.
Implanted devices are relative contraindications to CMR due to safety concerns and imaging artifacts. The presence of a cardiac implantable electronic device (CIED) was previously considered an absolute contraindication to MRI, but that has changed more recently. A growing body of literature in adults has demonstrated safety in scanning patients with non-MRI compatible devices [16], and there are data showing safety in children with congenital heart disease [17]. Newer MRI-conditional devices are also in use. In patients with hypoplastic left heart syndrome there is a 13% incidence of pacemaker placement by 8.5 years post Fontan [18]. In our study sample, 4.2% of enrolled patients had a pacemaker or ICD, and 7.6% had any device or metal fragment that prevented CMR exam. This lower incidence of pacemaker placement compared to published data may reflect a higher proportion of patients with pacemakers who were eligible to participate in SVR III, but did not enroll. This may also reflect a higher incidence of atrioventricular block in other forms of single ventricle CHD. Imaging susceptibility artifact leading to non-interpretable data remains a potential problem with CIEDs.
A recent single center review documented 20% anesthesia use in patients undergoing CMR exam for any reason at age 10 years [6]. In the current study of children 10 to 12.5 years of age, CMR could not be performed due to the need for anesthesia or sedation in 5.9% of subjects, a lower percentage than indicated in the earlier report. A higher incidence of developmental delay and/or behavioral difficulties in this patient population is a risk factor for need of sedation/anesthesia. While earlier work in this cohort demonstrated an increased percentage of participants at 6 years with scores in the at-risk or impaired range on measures of adaptive skills than expected for the population norms, there was a relatively low rate of SVR III participants who were unable to participate in the CMR due to lack of sedation/anesthesia. The lower percentage of subjects requiring sedation in this study could reflect advances in behavioral or CMR scanning techniques for helping children cooperate with non-sedated MRI [5]. These techniques, such as use of child life, movies and other distractions are anecdotally leading to decreased use of sedation/anesthesia for children in MRI at many centers.
CMR in younger children without anesthesia may require free breathing techniques, as many children are unable to comply with breath holding through the entire scan. Furthermore, children undergoing CMR under general anesthesia often require similar free breathing techniques to minimize the use of paralysis and ventilator pauses during an exam. While CMR more frequently required a breath hold for adequate imaging in the past, there are free breathing techniques in standard clinical use including multiple signal averaging and accelerated acquisition using parallel imaging, as well as emerging techniques such as real time CMR [19, 20] and four-dimensional imaging with cardiac registration [21,22,23]. As described in the methods, our protocol included accelerated image acquisition using parallel imaging and multiple signal averages for free breathing sequences. A total exam time of less than one hour and anatomic/functional assessment in 20 min is easily achievable. The vision for the future of CMR comprises rapid four-dimensional acquisitions with motion compensation, including in awake children less than 10 years of age and infants [21, 24], which will continue to break down barriers to CMR use in congenital heart disease research.
Study quality was adequate for measuring RVEF as the primary outcome using a free breathing CMR exam technique. Common artifacts experienced during the study included metallic susceptibility artifact, motion artifact, artifact from the MR environment (radiofrequency artifact) and phase contrast velocity encoding that was too low for the velocity of blood flow (aliasing). Older stainless steel coils and other implanted devices cause large susceptibility or “blooming” artifacts that obliterate much of the chest anatomy and were the most common reason for the inability to measure ejection fraction [25, 26]. Newer platinum, nitinol and alloy coils or stents cause no more than a local artifact and do not typically prevent the performance of quantitative measurements [27,28,29,30].
Our study is limited by potential selection bias in our long-term follow up of this specific cohort. Patients who were eligible but not enrolled may have been sicker, had developmental delays, genetic syndromes or had known implantable devices that resulted in the patients and families declining participation. There is also a survival bias in our follow up cohort, and it is possible that the patients who were transplanted or died could have had more difficult, lengthy, or poor-quality studies. This research protocol was designed for efficient CMR exams and not for evaluating accuracy or reproducibility of the volumetric analysis; however, it is important to note the protocol follows current guidelines for CMR use in children and adults with congenital heart disease 7. A minority of these studies were done for clinical indications that included contrast (16% of total studies) or sedation (5.6% of total studies), which often leads to lengthier examinations. These current data do not assess the ability of this patient population to tolerate longer, clinically indicated CMR exams without sedation or anesthesia.
Conclusion
In this prospective, longitudinal follow up study of the single ventricle reconstruction trial participants, use of CMR as a primary outcome measure was successful in 71% of participants. Cine imaging necessary for measuring ejection fraction could be obtained in ≤ 20 min. The most common reasons for inability to obtain this measure were related to susceptibility artifact from metallic implants and inability to tolerate the exam without sedation or anesthesia. These data are useful for feasibility planning of CMR in pediatric clinical trials, which will likely improve with the use of non-ferrous implantable devices, changes in use of CMR for patients with CIEDs, and continued evolution of CMR towards shorter, motion compensated examinations.
References
Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, Goldberg CS, Tabbutt S, Frommelt PC, Ghanayem NS, Laussen PC, Rhodes JF, Lewis AB, Mital S, Ravishankar C, Williams IA, Dunbar-Masterson C, Atz AM, Colan S, Minich LL, Pizarro C, Kanter KR, Jaggers J, Jacobs JP, Krawczeski CD, Pike N, McCrindle BW, Virzi L, Gaynor JW, Pediatric Heart Network I (2010) Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med 362:1980–1992
Newburger JW, Sleeper LA, Frommelt PC, Pearson GD, Mahle WT, Chen S, Dunbar-Masterson C, Mital S, Williams IA, Ghanayem NS, Goldberg CS, Jacobs JP, Krawczeski CD, Lewis AB, Pasquali SK, Pizarro C, Gruber PJ, Atz AM, Khaikin S, Gaynor JW, Ohye RG, Pediatric Heart Network I (2014) Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial. Circulation 129:2013–2020
Margossian R, Schwartz ML, Prakash A, Wruck L, Colan SD, Atz AM, Bradley TJ, Fogel MA, Hurwitz LM, Marcus E, Powell AJ, Printz BF, Puchalski MD, Rychik J, Shirali G, Williams R, Yoo SJ, Geva T, Pediatric Heart Network I (2009) Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study). Am J Cardiol 104:419–428
Pattynama PM, De Roos A, Van der Wall EE, Van Voorthuisen AE (1994) Evaluation of cardiac function with magnetic resonance imaging. Am Heart J 128:595–607
Dong SZ, Zhu M, Bulas D (2019) Techniques for minimizing sedation in pediatric MRI. J Magn Reson Imaging 50:1047–1054
Jung D, Wood J, Gloer K, Hernandez GI, Matich S, Goodarzian F, Yanofsky S, Ross PA, Detterich J (2016) Cardiac magnetic resonance imaging can be performed without the use of anesthesia in patients 7–10 years of age with child life support and MRI video goggles. J Cardiovasc Magn Reson 18:O120
Fratz S, Chung T, Greil GF, Samyn MM, Taylor AM, ValsangiacomoBuechel ER, Yoo SJ, Powell AJ (2013) Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson 15:51
Goldberg CS, Gaynor JW, Mahle WT, Ravishankar C, Frommelt P, Ilardi D, Bellinger D, Paridon S, Taylor M, Hill KD, Minich LL, Schwartz S, Afton K, Lamberti M, Trachtenberg FL, Gongwer R, Atz A, Burns KM, Chowdhury S, Cnota J, Detterich J, Frommelt M, Jacobs JP, Miller TA, Ohye RG, Pizarro C, Shah A, Walters P, Pediatric Heart Network I (2022) Newburger JW The pediatric heart network’s study on long-term outcomes of children with HLHS and the impact of Norwood Shunt type in the single ventricle reconstruction trial cohort (SVRIII): Design and adaptations. Am Heart J 254:216–227
Frommelt PC, Gerstenberger E, Cnota JF, Cohen MS, Gorentz J, Hill KD, John JB, Levine JC, Lu J, Mahle WT, McCandless RT, Mertens L, Pearson GD, Spencer C, Thacker D, Williams IA, Wong PC, Newburger JW, Pediatric Heart Network I (2014) Impact of initial shunt type on cardiac size and function in children with single right ventricle anomalies before the Fontan procedure: the single ventricle reconstruction extension trial. J Am Coll Cardiol 64:2026–2035
Aly S, Grosse-Wortmann L (2021) A novel approach to cardiovascular magnetic resonance imaging in young children without sedation. Eur J Radiol 144:110005
Fogel MA, Weinberg PM, Parave E, Harris C, Montenegro L, Harris MA, Concepcion M (2008) Deep sedation for cardiac magnetic resonance imaging: a comparison with cardiac anesthesia. J Pediatr 152:534–9 539.e1
Kharabish A, Mkrtchyan N, Meierhofer C, Martinoff S, Ewert P, Stern H, Fratz S (2016) Cardiovascular magnetic resonance is successfully feasible in many patients aged 3 to 8years without general anesthesia or sedation. J Clin Anesth 34:11–14
Majima N, Kagawa T, Suzuki T, Onishi H, Ikejima N (2010) General anesthesia for pediatric cardiac magnetic resonance imaging. Masui 59:935–939
Newburger JW, Sleeper LA, Bellinger DC, Goldberg CS, Tabbutt S, Lu M, Mussatto KA, Williams IA, Gustafson KE, Mital S, Pike N, Sood E, Mahle WT, Cooper DS, Dunbar-Masterson C, Krawczeski CD, Lewis A, Menon SC, Pemberton VL, Ravishankar C, Atz TW, Ohye RG, Gaynor JW, Pediatric Heart Network I (2012) Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: the single ventricle reconstruction trial. Circulation 125:2081–2091
Gerstle M, Beebe DW, Drotar D, Cassedy A, Marino BS (2016) Executive functioning and school performance among pediatric survivors of complex congenital heart disease. J Pediatr 173:154–159
Gupta SK, Ya’qoub L, Wimmer AP, Fisher S, Saeed IM (2020) Safety and clinical impact of MRI in patients with Non-MRI-conditional cardiac devices. Radiol Cardiothorac Imaging 2:e200086
Gakenheimer-Smith L, Etheridge SP, Niu MC, Ou Z, Presson AP, Whitaker P, Su J, Puchalski MD, Asaki SY, Pilcher T (2020) MRI in pediatric and congenital heart disease patients with CIEDs and epicardial or abandoned leads. Pacing Clin Electrophysiol 43:797–804
Williams RV, Travison T, Kaltman JR, Cecchin F, Colan SD, Idriss SF, Lu M, Margossian R, Reed JH, Silver ES, Stephenson EA, Vetter VL, Pediatric Heart Network I (2013) Vetter VL Comparison of Fontan survivors with and without pacemakers: a report from the Pediatric Heart Network Fontan Cross-Sectional Study. Congenit Heart Dis 8:32–39
Allen BD, Carr M, Botelho MP, Rahsepar AA, Markl M, Zenge MO, Schmidt M, Nadar MS, Spottiswoode B, Collins JD, Carr JC (2016) Highly accelerated cardiac MRI using iterative SENSE reconstruction: initial clinical experience. Int J Cardiovasc Imaging 32:955–963
Nayak KS, Lim Y, Campbell-Washburn AE, Steeden J (2022) Real-time magnetic resonance imaging. J Magn Reson Imaging 55:81–99
Lai LM, Cheng JY, Alley MT, Zhang T, Lustig M, Vasanawala SS (2017) Feasibility of ferumoxytol-enhanced neonatal and young infant cardiac MRI without general anesthesia. J Magn Reson Imaging 45:1407–1418
Nguyen KL, Han F, Zhou Z, Brunengraber DZ, Ayad I, Levi DS, Satou GM, Reemtsen BL, Hu P, Finn JP (2017) 4D MUSIC CMR: value-based imaging of neonates and infants with congenital heart disease. J Cardiovasc Magn Reson 19:40
Zhou Z, Han F, Rapacchi S, Nguyen KL, Brunengraber DZ, Kim GJ, Finn JP, Hu P (2017) Accelerated ferumoxytol-enhanced 4D multiphase, steady-state imaging with contrast enhancement (MUSIC) cardiovascular MRI: validation in pediatric congenital heart disease. NMR Biomed 30:e3663
Nguyen KL, Ghosh RM, Griffin LM, Yoshida T, Bedayat A, Rigsby CK, Fogel MA, Whitehead KK, Hu P, Finn JP (2021) Four-dimensional multiphase steady-state mri with ferumoxytol enhancement: early multicenter feasibility in pediatric congenital heart disease. Radiology 300:162–173
van der Graaf AW, Bhagirath P, Ghoerbien S, Gotte MJ (2014) Cardiac magnetic resonance imaging: artefacts for clinicians. Neth Heart J 22:542–549
Garg R, Powell AJ, Sena L, Marshall AC, Geva T (2005) Effects of metallic implants on magnetic resonance imaging evaluation of fontan palliation. Am J Cardiol 95:688–691
Breuckmann F, Nassenstein K, Boese D, Opherk D, Quick HH, Barkhausen J, Erbel R (2006) Successful nitinol stent implantation in a large coronary aneurysm: post-interventional patency assessment by magnetic resonance imaging. Int J Cardiovasc Imaging 22:501–505
Melzer M, Konak S, Bertsch T (2004) Nitinol in magnetic resonance imaging. Minim Invasive Ther Allied Technol 13:261–271
Hagspiel KD, Leung DA, Nandalur KR, Angle JF, Dulai HS, Spinosa DJ, Matsumoto AH, Christopher JM, Ahmed H, Berr SS (2005) Contrast-enhanced MR angiography at 1.5 T after implantation of platinum stents: in vitro and in vivo comparison with conventional stent designs. AJR Am J Roentgenol 184:288–294
Avitabile CM, Harris MA, Doddasomayajula RS, Chopski SG, Gillespie MJ, Dori Y, Glatz AC, Fogel MA, Whitehead KK (2018) Accuracy of phase-contrast velocity mapping proximal and distal to stent artifact during cardiac magnetic resonance imaging. Am J Cardiol 121:1634–1638
Acknowledgements
Long term Outcomes of Children with HLHS and the Impact of Norwood Shunt Type (SVRIII): National Heart, Lung, and Blood Institute: Gail Pearson, Victoria Pemberton, Kristin Burns, Mario Stylianou Protocol Chair: University of Texas Southwestern Medical Center, Lynn Mahony Data Coordinating Center: Carelon Research (New England Research Institutes), Felicia Trachtenberg (PI), Julie Miller (PI), Melissa Lamberti, Russell Gongwer, Allison Crosby-Thompson, Melissa Allen, Ayesha Amarnath, Bahar Coley, Lozan Eyob, Beverly Slayton, Tanya Olesker*, Chenwei Hu, Danielle Hollenbeck-Pringle*, Paul Stark*, Barbara Winrich*, Shreya Divatia*, Andrew Morrison*, Chitra Kinhikar*; Core Clinical Site Investigators: Boston Children’s Hospital, Jane W. Newburger (PI), David Bellinger, Ashwin Prakash, Jamie Levine, Jonathan Rhodes, Carolyn Dunbar-Masterson, Lisa Jean Buckley; Children’s Hospital of New York, Amee Shah (PI), Margaret Challenger, Annette Zygmunt, Robert Garofano, Kathleen Gilmartin*, Katrina Golub, Rosalind Korsin, Chanel Rojas; Children’s Hospital of Philadelphia, J. William Gaynor (PI), Chitra Ravishankar (PI), Katherine Lupton, Tonia Morrison, Meryl Cohen, Stephen Paridon, Thomas Flynn, Kevin Whitehead, Donna Sylvester*, Somaly Srey, Angela Winters; Cincinnati Children’s Medical Center, James Cnota (PI), Teresa Barnard§, Michelle Hamstra*, Kathleen Rathge; Duke University Medical Center, Kevin Hill (PI) Jennifer Li, Michael Campbell, Michael Carboni, Jennifer Martin, Melanie Simms, Kathryn Gustafson, Kimberly Carroll*, Mingfen Xu; Medical University of South Carolina, Andrew Atz (PI), Shahryar Chowdhury, Kimberly McHugh, Mary Kral, Ryan Butts*, Geoffrey Forbus, Arni Nutting, Carolyn Taylor, Shameeka Bowman*, Kaylan Chundru*, Layla Al Sarraf, Mary Freeman, Patricia Infinger*; Primary Children’s Hospital and the University of Utah, Salt Lake City, Utah Thomas Miller (Co-PI, now at MMC), L. LuAnn Minich (Co-PI), Philip Burch*, Richard Williams, Sean Cunningham, Linda Lambert, Bergen Lindauer*, Marian Shearrow: Hospital for Sick Children, Toronto, Steven Schwartz (PI), Brian McCrindle, Martha Rolland, Patricia Walter, Patricia Arseneau, Wei Hui, Susan Iori, Cameron Slorach, Andreea Dragulescu, Renee Sananes, Mike Seed; University of Michigan, University of Michigan, Caren S. Goldberg (PI), Mark Russell, Katherine Afton, Chelsea Stewart, Kimberly Heinrich, Jimmy Lu, Adam Dorfman Children’s Hospital of Wisconsin, Michele Frommelt (PI), Kathy Mussatto*, Peter Frommelt, Nancy Ghanayem*, Michelle Loman, Michelle Otto, Jessica Stelter, Jennifer Dixon, Andrea Moker, Michael Danduran, Stacy Leibham, Megan Schoessling; Auxiliary Sites: Children’s Hospital Los Angeles, Jon Detterich (PI), Alan Lewis, Sharon O’Neill, Hesham Mahmoud*, Taqwa Ramadan*; John Hopkins All Children’s Heart Institute, Marguerite Crawford (PI), Jeffrey P. Jacobs*, Jade Hanson, Lexie Dallas, Dawn Morelli*; Emory University, William Mahle (PI), Dawn Ilardi, Ritu Sachdeva, Timothy Slesnick, William Border, Courtney Fyock*, Susie Gentry*, Rae Ashley*, Amanda Graham*, Leslie Smitley*; Nemours Cardiac Center, Christian Pizarro, Jeanne Baffa, Michael McCulloch, Brad Robinson, Erica Sood, Carol Prospero; Echocardiography core laboratory: Children’s Hospital of Wisconsin, Peter Frommelt, Jessica Stelter, Meagan Schoessling; Exercise Core Laboratory: Children’s Hospital of Philadelphia, Stephen Paridon MRI Core Laboratory: Cincinnati Children’s Medical Center, Michael Taylor, Joshua Germann, Peace Madueme Protocol Review Committee: Michael Artman (Former Chair), Timothy Feltes, Jogarao Gobburu, Sally Hunsberger, Paul Matherne Data and Safety Monitoring Board: Dianne Atkins, Preetha Balakrishnan, Craig Broberg, David J. Driscoll, David J. Gordon (Former Executive Secretary), Frank Evans (DSMB Executive Secretary), Sally A. Hunsberger, Liza-Marie Johnson, Mark Galantowicz , Thomas J. Knight, John Kugler (Former Chair), Paul Lipkin, J. Philip Saul (DSMB Chair), Holly Taylor *No longer with institution §Deceased.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium. This study was funded by NIH 1 U10 HL068270, Pediatric Heart Network - NHLBI (Goldberg).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
MC Consultant for Longeveron, JD Scientific Advisory Board for Alcor Scientific.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Detterich, J., Taylor, M.D., Slesnick, T.C. et al. Cardiac Magnetic Resonance Imaging to Determine Single Ventricle Function in a Pediatric Population is Feasible in a Large Trial Setting: Experience from the Single Ventricle Reconstruction Trial Longitudinal Follow up. Pediatr Cardiol 44, 1454–1461 (2023). https://doi.org/10.1007/s00246-023-03216-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-023-03216-8