Abstract
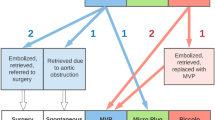
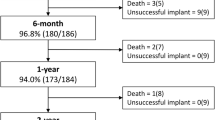
To evaluate short-term procedural outcomes and safety for infants < 2.5 kg who underwent catheterization with intended patent ductus arteriosus (PDA) device closure in a multi-center registry, as performance of this procedure becomes widespread. A multi-center retrospective review was performed using data from the Congenital Cardiac Catheterization Project on Outcomes (C3PO) registry. Data were collected for all intended cases of PDA closure in infants < 2.5 kg from April 2019 to December 2020 at 13 participating sites. Successful device closure was defined as device placement at the conclusion of the catheterization. Procedural outcomes and adverse events (AE) were described, and associations between patient characteristics, procedural outcomes and AEs were analyzed. During the study period, 300 cases were performed with a median weight of 1.0 kg (range 0.7–2.4). Successful device closure was achieved in 98.7% of cases with a 1.7% incidence of level 4/5 AEs, including one periprocedural mortality. Neither failed device placement nor adverse events were significantly associated with patient age, weight or institutional volume. Higher incidence of adverse events associated with patients who had non-cardiac problems (p = 0.017) and cases with multiple devices attempted (p = 0.064). Transcatheter PDA closure in small infants can be performed with excellent short-term outcomes and safety across institutions with variable case volume.



Similar content being viewed by others
Abbreviations
- AE:
-
Adverse event
- C3PO:
-
Congenital Cardiac Catheterization Project on Outcomes
- FDA:
-
Food and Drug Administration
- IQR:
-
Inter-quartile range
- PDA:
-
Patent ductus arteriosus
References
Porstmann W, Wierny L, Warnke H et al (1971) Catheter closure of patent ductus arteriosus. 62 cases treated without thoracotomy. Radiol Clin North Am 9:203–218
Wierny L, Plass R, Porstmann W (1986) Transluminal closure of patent ductus arteriosus: long-term results of 208 cases treated without thoracotomy. Cardiovasc Intervent Radiol 9:279–285. https://doi.org/10.1007/BF02577958
Bischoff AR, Jasani B, Sathanandam SK et al (2021) Percutaneous closure of patent ductus arteriosus in infants 1.5 kg or less: a meta-analysis. J Pediatr 230:84–92. https://doi.org/10.1016/j.jpeds.2020.10.035
Kobayashi D, Salem MM, Forbes TJ et al (2019) Results of the combined U.S. multicenter postapproval study of the Nit-Occlud PDA device for percutaneous closure of patent ductus arteriosus. Catheter Cardiovasc Interv 93:645–651. https://doi.org/10.1002/ccd.27995
El-Said HG, Bratincsak A, Foerster SR et al (2013) Safety of percutaneous patent ductus arteriosus closure: an unselected multicenter population experience. J Am Heart Assoc 2:e000424. https://doi.org/10.1161/JAHA.113.000424
Magee AG, Huggon IC, Seed PT et al (2001) Transcatheter coil occlusion of the arterial duct: results of the European registry. Eur Heart J 22:1817–1821. https://doi.org/10.1053/euhj.2001.2605
Masura J, Tittel P, Gavora P, Podnar T (2006) Long-term outcome of transcatheter patent ductus arteriosus closure using Amplatzer duct occluders. Am Heart J 151:755. https://doi.org/10.1016/j.ahj.2005.12.010
Masura J, Walsh KP, Thanopoulous B et al (1998) Catheter closure of moderate- to large-sized patent ductus arteriosus using the new Amplatzer duct occluder: immediate and short-term results. J Am Coll Cardiol 31:878–882. https://doi.org/10.1016/S0735-1097(98)00013-8
Pass RH, Hijazi Z, Hsu DT et al (2004) Multicenter USA amplatzer patent ductus arteriosus occlusion device trial: initial and one-year results. J Am Coll Cardiol 44:513–519. https://doi.org/10.1016/j.jacc.2004.03.074
Jobe AH (2006) The new BPD. Neoreviews 7:e531–e545. https://doi.org/10.1542/neo.7-10-e531
Schena F, Francescato G, Cappelleri A et al (2015) Association between hemodynamically significant patent ductus arteriosus and bronchopulmonary dysplasia. J Pediatr 166:1488–1492. https://doi.org/10.1016/j.jpeds.2015.03.012
Slutsky AS, Ranieri VM (2013) Ventilator-induced lung injury. N Engl J Med 22369:2126–2136. https://doi.org/10.1056/NEJMra1208707
Abu Hazeem AA, Gillespie MJ, Thun H et al (2013) Percutaneous closure of patent ductus arteriosus in small infants with significant lung disease may offer faster recovery of respiratory function when compared to surgical ligation. Catheter Cardiovasc Interv 82:526–533. https://doi.org/10.1002/ccd.25032
Clement WA, El-Hakim H, Phillipos EZ, Coté JJ (2008) Unilateral vocal cord paralysis following patent ductus arteriosus ligation in extremely low-birth-weight infants. Arch Otolaryngol - Head Neck Surg 134:28–33. https://doi.org/10.1001/archoto.2007.2
Teixeira LS, Shivananda SP, Stephens D et al (2008) Postoperative cardiorespiratory instability following ligation of the preterm ductus arteriosus is related to early need for intervention. J Perinatol 28:803–810. https://doi.org/10.1038/jp.2008.101
Sathanandam S, Balduf K, Chilakala S et al (2019) Role of Transcatheter patent ductus arteriosus closure in extremely low birth weight infants. Catheter Cardiovasc Interv 93:89–96
Zahn EM, Peck D, Phillips A et al (2016) Transcatheter closure of patent ductus arteriosus in extremely premature newborns: early results and midterm follow-up. JACC Cardiovasc Interv 9:2429–2437. https://doi.org/10.1016/j.jcin.2016.09.019
Sathanandam S, Justino H, Waller BR et al (2017) Initial clinical experience with the Medtronic Micro Vascular Plug™ in transcatheter occlusion of PDAs in extremely premature infants. Catheter Cardiovasc Interv 89:1051–1058. https://doi.org/10.1002/ccd.26878
Sathanandam SK, Gutfinger D, O’Brien L et al (2020) Amplatzer Piccolo Occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients ≥700 grams. Catheter Cardiovasc Interv 96:1266–1276. https://doi.org/10.1002/ccd.28973
Quinn BP, Cevallos P, Armstrong A et al (2020) Longitudinal improvements in radiation exposure in cardiac catheterization for congenital heart disease: a prospective multicenter C3PO-QI study. Circ Cardiovasc Interv 13:e008172. https://doi.org/10.1161/CIRCINTERVENTIONS.119.008172
Quinn BP, Yeh M, Gauvreau K et al (2022) Procedural risk in Congenital Cardiac Catheterization (PREDIC3T). J Am Heart Assoc 11:22832. https://doi.org/10.1161/JAHA.121.022832
Goldstein BH, Bergersen L, Armstrong AK et al (2020) Adverse events, radiation exposure, and reinterventions following transcatheter pulmonary valve replacement. J Am Coll Cardiol 75:363–376. https://doi.org/10.1016/j.jacc.2019.11.042
Bergersen L, Gauvreau K, Foerster SR et al (2011) Catheterization for Congenital Heart Disease Adjustment for Risk Method (CHARM). JACC Cardiovasc Interv 4:1037–1046. https://doi.org/10.1016/j.jcin.2011.05.021
Kuntz MT, Staffa SJ, Graham D et al (2022) Trend and outcomes for surgical versus transcatheter patent ductus arteriosus closure in neonates and infants at US children’s hospitals. J Am Heart Assoc 11:e022776. https://doi.org/10.1161/JAHA.121.022776
Bentham J, Meur S, Hudsmith L et al (2011) Echocardiographically guided catheter closure of arterial ducts in small preterm infants on the neonatal intensive care unit. Catheter Cardiovasc Interv 77:409–415. https://doi.org/10.1002/ccd.22637
Backes CH, Cheatham SL, Deyo GM et al (2016) Percutaneous patent ductus arteriosus (PDA) closure in very preterm infants: feasibility and complications. J Am Heart Assoc 5:e002923. https://doi.org/10.1161/JAHA.115.002923
Markush D, Tsing JC, Gupta S et al (2021) Fate of the left pulmonary artery and thoracic aorta after transcatheter patent ductus arteriosus closure in low birth weight premature infants. Pediatr Cardiol 42:628–636. https://doi.org/10.1007/s00246-020-02523-8
Funding
No funding was received to support this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. OB, TG, MT: responsible for the primary design of the study. DB, MB, BB, RC, HE, MF, SF, BG, RH, DJ, RL, MO, GR, SSa, SSh, WW: contributions to the study design and its completion. OB, GR: responsible for material preparation and data collection. GR: primarily responsible for the data analysis with contributions from OB, MT, TG. The first draft of the manuscript was written and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
DB: Abbott (proctor); BG: Medtronic (consultant); TG: Abbott (proctor); SSa: Abbott (proctor, consultant and research grant); SSh: Abbott (proctor, consultant), Medtronic (proctor, consultant). No other authors have any relevant disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barry, O.M., Gudausky, T.M., Balzer, D.T. et al. Safety and Short-Term Outcomes for Infants < 2.5 kg Undergoing PDA Device Closure: A C3PO Registry Study. Pediatr Cardiol 44, 1406–1413 (2023). https://doi.org/10.1007/s00246-023-03147-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-023-03147-4