Abstract
There is a paucity of longitudinal data on cardiac outcomes in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. We aimed to investigate the longitudinal cardiovascular outcomes in MIS-C. PubMed and EMBASE were searched through May 2022. Observational studies were included, reporting mid-term (≥ 3 months) outcomes in children (aged < 21) with MIS-C. Data were extracted by two researchers. Longitudinal outcomes were synthesized by a one-group meta-analysis using a random-effects model. Eleven studies with a follow-up period (3 months to 1 year) were identified, including 547 MIS-C patients. The mortality was 2.5% (95% CI 1.3–4.9). The majority of left ventricular (LV) systolic dysfunction present in 46.8% (95% CI 32.7–61.3) in the acute phase resolved by 3 months, and the prevalence of LV systolic dysfunction was 1.7% (95% CI 0.5–5.7) and 2.1% (95% CI 0.8–5.4) at 3 month and 6 month follow-up, respectively. Additionally, the persistent LV systolic dysfunction in the small population was mild. However, coronary abnormalities such as coronary artery dilatation or aneurysms, seen in 23.7% (95% CI 17.7–31.1) at baseline, persisted in 4.7% (95% CI 1.5–14.3) at 3 months and 5.2% (95% CI 3.0–8.9) at 6 months. Mitral regurgitation (MR), which was observed in 56.6% (95% CI 27.7–81.6) at baseline, also persisted in 7.5% at 6 months. In conclusion, our study demonstrated largely favorable cardiac outcomes, suggesting resolution of LV systolic dysfunction in the majority of cases. However, coronary abnormalities and MR persisted in a subset of patients at mid-term follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease 2019 (COVID‐19) has been a global pandemic since December 2019. Initial studies reported that children experienced mild manifestations of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in contrast to adults [1]. However, in April 2020, a novel post-infectious hyperinflammatory syndrome associated with COVID-19, now termed as multisystem inflammatory syndrome in children (MIS-C) or pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2, was identified [2, 3].
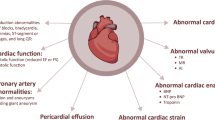
The clinical features in the acute phase and short-term outcomes of MIS-C have been described previously. Cardiac involvement occurs in approximately 80% of children with MIS-C, including left ventricular (LV) dysfunction, coronary abnormalities, pericardial effusions, arrhythmias, and conduction abnormalities [4,5,6,7,8,9]. Up to 50% of MIS-C patients exhibit LV systolic and/or diastolic dysfunction, and 10–20% of cases develop coronary artery dilatation and/or aneurysms [2, 10, 11]. Previous reports suggest that most of the patients with severe cardiac involvement recovered from LV systolic dysfunction and coronary abnormalities in the acute phase [12,13,14]. Recently, several single-center studies have revealed that mid-term outcomes of MIS-C are largely reassuring [15,16,17,18,19,20]. However, the reported incidence of persistent cardiac manifestations, such as LV systolic dysfunction and coronary abnormalities, varies due to the small sample size in each study. In addition, longitudinal outcomes of various cardiac manifestations in MIS-C remain unclear, including persistent LV diastolic dysfunction and mitral regurgitation (MR).
Elucidating the prevalence and time-course of cardiac abnormalities at mid-term follow-up will help in understanding the long-term outcomes and determining the management plans for MIS-C. Thus, we conducted a systematic review and meta-analysis to clarify mid-term cardiovascular outcomes in patients with MIS-C.
Materials and Methods
Search Strategy
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines [21, 22]. All observational studies which included children with MIS-C associated with COVID-19 were identified using a 2-level search strategy. Databases including PubMed and EMBASE were searched through May 5th, 2022. Relevant studies were identified through a manual search of secondary sources including references of initially identified articles, reviews, and commentaries. All references were downloaded for consolidation, elimination of duplicates, and further analyses. Search terms included COVID-19, SARS-CoV-2, coronavirus, MIS-C, multisystem inflammatory syndrome in children, PIMS, PIMS-TS, pediatric inflammatory multisystem syndrome, children, child, pediatric, young people, long-term, mid-term, outcomes, and follow-up. We did not apply language limitations.
Study Selection
The following inclusion criteria were applied: (1) the study design was an observational study that was published in a peer-reviewed journal, (2) the study population was children < 21 years of age, meeting the diagnostic criteria for MIS-C with evidence of SARS-CoV-2 infection, (3) the lengths of follow-up satisfied mid-term (≥ 3 months) evaluation. The diagnosis of MIS-C was based on the Center for Disease Control and Prevention definition or the Royal College of Paediatrics and Child Health diagnostic criteria for PIMS-TS [23, 24]. Studies reporting data at either 3 months, 6 months or 1 year follow-up were eligible, while studies with the lengths of follow-up < 3 months were excluded. Articles that do not contain original data of the patients such as guideline, editorial, and review were excluded from the secondary review.
Two independent authors (J.Y. and K.M.) reviewed the search results separately to select the studies based on the inclusion and exclusion criteria. Disagreements were resolved by consensus with a third author (T.K.).
Data Extraction and Quality Assessment
Two authors (J.Y. and K.M.) performed the data extraction independently. The following information was extracted: study details (first author, date of publication, study location, and study design), patient demographic and clinical characteristics (number of patients, age, gender, race/ethnicity, laboratory values, and treatments), and mid-term outcomes (death, echocardiographic and cardiac magnetic resonance [CMR] findings). Discrepancies regarding the extracted data were resolved through discussion and consensus with a third author (T.K.). Echocardiographic findings including LV function, coronary abnormalities, MR, and pericardial effusion were collected serially from baseline to 3 months, 6 months, and 1 year follow-up. LV systolic dysfunction was defined as left ventricular ejection fraction (LVEF) < 55%. LV diastolic dysfunction was defined as at least 2 parameters (E/A, e’, or E/e’) being abnormal. The mitral inflow E/A Doppler profile was considered abnormal if the E and A waves were fused or if the E/A ratio had a Boston Children’s Hospital Z score > 2.0. The e’ velocity and E/e’ ratio, either septal or lateral, were considered abnormal if either had a Boston Children’s Hospital Z score > 2.0. Coronary abnormalities were defined by z scores as follows: normal < 2, dilation 2 to < 2.5, aneurysm ≥ 2.5 [25]. The risk of bias for the prevalence studies for each retrospective cohort study was assessed using the Newcastle–Ottawa Assessment Scale [26].
Statistical Analysis
We performed one-group meta-analysis in a random-effects model using the DerSimonian-Laird method for continuous values and Wald method for discrete values. Comprehensive Meta-Analysis version 2 (available from https://www.meta-analysis.com/index.php?cart=BTEJ5270189) and OpenMetaAnalyst version 12.11.14 (available from http://www.cebm.brown.edu/openmeta/) were used for statistical analysis. Continuous variables are expressed as the means ± standard deviations or medians (interquartile range), as appropriate for the data distribution. Categorical variables are expressed as frequencies and percentages. The I2 statistic was used to quantify heterogeneity, with I2 > 50% indicating substantial heterogeneity.
Results
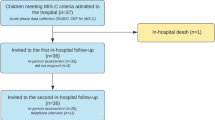
A total of 574 articles were identified through the initial database searching and the removal of duplicated items. After title and abstract screening, 190 full-text articles were assessed for eligibility and 179 articles were excluded based on lack of inclusion criteria such as the article type (editorials, reviews, systematic reviews, and meta-analyses), population (adult patients with COVID-19, cases without meeting the case definition for MIS-C), and articles without mid-term or long-term outcomes. Finally, 11 observational studies [15,16,17,18,19,20, 27,28,29,30,31] were included in our meta-analysis (Fig. 1).
Study Characteristics
The study and patient characteristics are summarized in Table 1. Across all 11 studies, a total of 547 patients with MIS-C were included. All the included studies were published in 2021 and 2022. Among these 11 studies, 6 studies were from the United States, 3 from the United Kingdom, and 2 from other countries (India and Pakistan). The follow-up timing varied up to 1 year; however, these studies all reported follow-up data in patients with MIS-C at 3 months or longer following initial diagnosis or discharge, including echocardiographic and CMR assessment. We synthesized outcomes at 3 months from 2 studies [27, 31] which explicitly included a follow-up at 3 months and 3 studies [17, 20, 30] which reported data at 100–105 days. We also synthesized outcomes at 6 months from 7 studies [16,17,18,19, 28, 29, 31] which included data at 6 months or 180 days follow-up. A summary of the risk of bias assessment for the prevalence studies for each retrospective cohort study is shown in Supplementary Table 1.
The pooled estimates from one-group meta-analysis in a random-effects model are presented in Table 2. The mean age at baseline was 9.5 [95% confidence interval (CI) 8.5–10.5; I2 = 79.8%] and males were 62.2% (95% CI 57.9–66.5; I2 = 0%). The pooled proportions of Hispanic, Black, White, and Asian cases were 18.5% (95% CI 8.1–28.9; I2 = 86.1%), 35.2% (95% CI 21.6–48.9; I2 = 87.4%), 18.2% (95% C, 9.6–26.9; I2 = 80.7%) and 8.0% (95% CI 3.1–12.9; I2 = 68.1%), respectively. Laboratory findings showed marked elevations in D-dimer, inflammatory markers including C-reactive protein and ferritin, and cardiac markers including N-terminal proB-type natriuretic peptide and troponin T (Table 2).
The pooled estimates regarding detailed information of treatment for MIS-C are shown in Table 2. The most common immunosuppressant was intravenous immunoglobulin (IVIG) (84.6%; 95% CI 78.4–90.7; I2 = 77.2%), followed by systemic corticosteroids (72.8%; 95% CI 61.2–84.3; I2 = 94.4%). A range of anti-inflammatory biologics and antiviral agents were used including tocilizumab, anakinra, infliximab, and remdesivir. The majority of the patients received aspirin (85.3%; 95% CI 74.6–96.1; I2 = 85.3%) and anticoagulation (68.3%; 95% CI 47.0–89.5; I2 = 90.3%). About half of the patients required inotropes (50.1%; 95% CI 31.3–68.8; I2 = 0%; I2 = 88.4%) and intensive care unit admission (46.7%; 95% CI 26.9–67.7; I2 = 91.8%; I2 = 94.9%). Overall, 17.8% (95% CI 0.0–49.7; I2 = 94.9%) required mechanical ventilation, and 1.4% (95% CI 0.5–4.3; I2 = 0%) needed extracorporeal membrane oxygenation (ECMO) (Supplementary Fig. 1 and 2). The mortality rate was 2.5% (95% CI 1.3–4.9; I2 = 0%) (Supplementary Fig. 3).
Ventricular Dysfunction
Longitudinal echocardiographic findings of the included study are summarized in Table 3. At baseline, LV systolic dysfunction, defined as LVEF < 55%, was identified in 46.8% (95% CI 32.7–61.3; I2 = 87.4%) (Fig. 2) and the mean LV ejection fraction was 54.1% (95% CI 49.4–58.7; I2 = 95.6%) (Fig. 3). Three studies demonstrated that the prevalence of mild LV systolic dysfunction (LVEF 45–54%) was 62.8% (54/86) and the prevalence of moderate (LVEF 35–44%) or severe (LVEF < 35%) LV systolic dysfunction was 37.2% (32/86) at baseline. The longitudinal pooled prevalence of LV systolic dysfunction showed continued recovery in ventricular function from baseline to 6 months follow-up. The majority of the patients showed normalization of LV systolic dysfunction and the prevalence of LV systolic dysfunction was 1.7% (95% CI 0.5–5.7; I2 = 0%) at 3 months and 2.1% (95% CI 0.8–5.4; I2 = 0%) at 6 months (Fig. 2). All the persistent LV systolic dysfunction seen at 3 months and 6 months was mild, suggesting that there was no physiologically significant LV systolic dysfunction. At 3 months and 6 months follow-up, LVEF was 65.6% (95% CI 63.6–67.6; I2 = 83.5%) and 61.6% (95% CI 60.0–63.3; I2 = 81.9%), respectively (Fig. 3).
The incidence of LV diastolic dysfunction was calculated by summation because there were only 2 studies available [18, 28]. LV diastolic dysfunction was identified in 32% (32/100) at baseline and 4.1% (2/49) at 6 months.
Coronary Abnormalities
In the acute phase, the pooled prevalence of coronary abnormalities such as coronary artery dilation or aneurysms was 23.7% (95% CI 17.7–31.1; I2 = 68.5%). Many patients had resolution of coronary abnormalities in the acute period; however, coronary abnormalities were still observed in 4.7% (95% CI 1.5–14.3; I2 = 36.2%) of the patients at 3 months. Notably, coronary abnormalities persisted in 5.2% (95% CI 3.0–8.9; I2 = 0%) of the patients at 6 months (Fig. 4).
Mitral Regurgitation and Pericardial Effusion
At baseline, 56.6% (95% CI 27.7–81.6; I2 = 91.1%) had MR. Although MR resolved in some patients, MR persisted in 7.5% (95% CI 1.3–32.8; I2 = 73.2%) at 6 months (Fig. 5). The incidence of pericardial effusion was 32.1% (95% CI 15.8–54.3; I2 = 87.3%) in the acute phase. The majority of patients showed resolution of pericardial effusion seen in 2.5% (95% CI 0.6–9.4; I2 = 0%) at 6 months (Supplementary Fig. 4).
Discussion
The salient findings of our study can be summarized as follows: (1) The overwhelming majority of cases of LV systolic dysfunction present in the acute stages of MIS-C resolved completely by 3 months and the persistent LV systolic dysfunction in the small proportion of patients was very mild; (2) The prevalence of coronary abnormalities was 23.7% at baseline, while coronary abnormalities persisted in 4.7% and 5.3% of patients at 3 months and 6 months; (3) More than half of the patients had MR at baseline, which persisted in 7.5% at 6 months.
Our meta-analysis demonstrated that the majority of LV systolic dysfunction seen at baseline mostly resolved by 6 months and the persistent LV systolic dysfunction in the small proportion of patients was physiologically insignificant. Furthermore, Davies et al. [17] reported 1 year outcomes of 68 patients with MIS-C, showing that all of 39 patients (57.4%) with cardiac dysfunction at admission recovered by day 74. This finding suggests that mid-term myocardial function outcomes are largely reassuring. Matsubara et al. found evidence of myocardial strain abnormalities despite normal LVEF, which suggests occult or subclinical systolic dysfunction [13]. Sanil et al. found persistent myocardial strain abnormalities at 10 weeks follow-up, with those having worse strain being more likely to experience hypotension, acute myocardial injury, inotropic requirement, cardiogenic shock, ECMO support, and longer hospital stay [32]. A recent study provided reassurance using sensitive deformation parameters that there was a rapid recovery of diastolic dysfunction by 3 months, suggesting no persistent subclinical dysfunction after. However, the assessment of diastolic function in children is limited since it has poor interobserver agreement and can vary according to the volume status and valve competency, which makes the measurements unreliable and may have an adverse impact on interpretation [18]. Therefore, diastology is a concern in a subset of MIS-C patients, but more advanced functional imaging methods, such as strain, will better evaluate long-term findings. In addition to LV dysfunction, Farooqi et al. [16]. demonstrated that right ventricular (RV) dysfunction, seen in 35.6% of MIS-C patients at baseline, persisted in 6.5% and 4.2% of the patients at 1–4 months and 4–9 months follow-up, respectively. Ventricular systolic and diastolic dysfunction may be underestimated using only traditional echocardiographic measures. Furthermore, our study demonstrated that more than half of the patients had MR at baseline, which persisted in 7.5% at 6 months. MIS-C patients have been reported to show MR more than Kawasaki disease (KD) [13]. However, the mechanisms and clinical implications of MR in MIS-C remain unclear. Long-term clinical implications of LV myocardial strain abnormalities, diastolic dysfunction, RV dysfunction, and MR are currently understudied and need further investigation using speckle-tracking echocardiography and CMR imaging.
In addition, the underlying mechanisms as well as an accurate diagnosis and optimal follow-up of myocardial dysfunction in MIS-C remains poorly understood. According to the predominant hypothesis for the pathophysiology of MIS-C, a potential mechanism for myocardial dysfunction may be post-infectious myocarditis caused by a severe cytokine storm, which is supported by laboratory data such as elevated B-type natriuretic peptide and troponin, and reduced tissue deformation indices [13, 33, 34]. CMR remains the non-invasive reference standard for myocardial tissue characterization, particularly in myocarditis. However, there are only a limited number of CMR studies in children with MIS-C focusing on myocardial tissue characterization techniques and the extent of abnormalities seen varies across studies. Several studies have demonstrated the presence of myocardial edema, with prevalence as high as 44–75% of patients with MIS-C [19, 35]. A recent publication by the CARDOVID Registry, which is one of the largest CMR-based studies in patients with MIS-C, identified 22/110 (20%) of patients with evidence of late gadolinium enhancement (LGE), with the vast majority meeting CMR criteria for myocarditis. Extent or quantity of LGE, which represents replacement fibrosis or acute myocardial necrosis, has been shown to be a risk factor for long-term outcomes in viral myocarditis data [36]. Short-term findings assessed by CMR demonstrated myocardial deformation indices were in the normal range within a few weeks after the onset of MIS-C; however, lower strain parameters were found in a subset of children, suggestive of persistent subtle myocardial dysfunction [37]. Our echocardiography findings seem reassuring in terms of recovery from LV dysfunction; however, this may be due to underestimating true recovery as CMR can continue to show myocardial inflammation and decreased strain at follow-up. A recent statement from the American Heart Association proposed that CMR can be utilized to confirm the diagnosis of myocarditis in children and endomyocardial biopsy can be taken into consideration if necessary [38]. Markers of myocardial inflammation or edema by CMR are high signal intensity on T2-weighted imaging and markers of myocardial fibrosis are increased native T1 and extracellular volume fraction. Hence, CMR is useful for diagnosis as well as follow-up evaluation of myocardial function in MIS-C. Further longitudinal studies by the combination of echocardiography and CMR would help refine the clinical course of full myocardial recovery and establish appropriate follow-up in children with MIS-C.
In our study, we exhibited many of coronary abnormalities regressed at follow-up period; however, coronary abnormalities noted at 3 months persisted until 6 months and would likely be present over the long-term. Previous studies showed mainly favorable early and mid- to long-term outcomes with recovery of coronary abnormalities [13, 18, 39]. One-year outcomes of 68 patients with MIS-C showed that all the 19 patients (27.9%) with coronary aneurysms at admission had resolution by day 400 [17]. In contrast, although infrequent, severe coronary artery involvement such as progression of giant coronary aneurysms has been described to date [2, 3, 40,41,42,43]. Some previous studies separated persistent coronary ectasia which is defined as coronary dilatation without a segmental aneurysm from coronary aneurysms [13]. Given the persistent coronary artery involvement in MIS-C, we highlight the need for continued caution and cardiac evaluation with serial echocardiography at regular follow-up period to investigate the detailed characteristics of coronary abnormalities, including coronary dilatation, coronary ectasia, and coronary aneurysms. Future studies with standardized follow-up protocols, longer-term surveillance, and core laboratory interpretation are needed to identify the prevalence and progression of coronary abnormalities in MIS-C. Moreover, although MIS-C has some clinical similarities to KD, differences in long-term outcomes of coronary abnormalities between MIS-C and KD remain unclear. Persistent coronary artery aneurysms occur in about 5% of patients with KD, including about 1% developing giant coronary artery aneurysms [44]. Regression occurs in 75% of KD patients with coronary artery aneurysms and the initial diameter of coronary artery aneurysms has been shown to be predictive of outcomes [45, 46]. Further research is required to understand potentially variable long-term outcomes as well as pathophysiology of coronary abnormalities in MIS-C in comparison to KD as some of MIS-C patients also meet the diagnostic criteria of incomplete KD [5].
Arrhythmias and electrocardiographic changes have been reported in 30–70% of MIS-C patients [7, 47,48,49]. First-degree heart block was observed in 6–25% of patients more frequently than second- or third-degree heart block which was commonly associated with LV systolic dysfunction. Sinus bradycardia has also been reported [50, 51]. Patients with persistent ventricular tachyarrhythmias had evidence of LV systolic dysfunction, requiring ECMO support [52, 53]. Electrocardiographic changes included low QRS amplitude, ST segment changes, QT prolongation, and T-wave abnormalities [48]. Long-term data on arrhythmias and electrocardiographic changes in MIS-C are warranted to understand persistence or recurrence of these abnormalities.
There are no randomized controlled trials assessing the efficacy of treatment in patients with MIS-C. Current treatment protocols are based on management experiences with KD because of the similarities between MIS-C and KD. IVIG has been used most commonly in our study as well as the previous studies in approximately 70–100% of patients with MIS-C [54,55,56]. We showed that corticosteroids were also used commonly in our study. As for corticosteroids therapy, a previous study revealed that patients who received combination therapy of IVIG and corticosteroids were less likely to have LV dysfunction at 1–2 days after initial treatment [54]. However, an international observational cohort study did not detect any significant differences in LV dysfunction or coronary abnormalities between IVIG alone, corticosteroids alone, or IVIG plus combination [56]. Based on the results of those studies, IVIG and/or corticosteroids therapy are favored as first-line treatment, which can contribute to the improvement of LV dysfunction and recovery from coronary abnormalities after the acute phase of MIS-C [57]. Biologic agents, such as interleukin-1, interleukin-6, and tumor necrosis factor-α blockers, can be used as second-line immunomodulatory treatment in MIS-C that is refractory to IVIG and corticosteroids [58, 59]. The clinical role of antiviral therapy with remdesivir is limited because of the lack of efficacy and safety data in children, and is utilized for severe acute COVID-19 [60]. Due to the risk of thrombosis with MIS-C, antiplatelet therapy and anticoagulants should be considered in MIS-C patients especially with LV dysfunction or coronary artery aneurysms [61].
This study had several limitations to be noted. First, the description of follow-up timing was variable and inconsistent between studies, which made it difficult to precisely compare the proportion of patients with residual cardiac abnormalities. Second, the available studies were observational studies, which are subject to methodological biases or reporting biases. Third, several variables, such as LV diastolic dysfunction and strain parameters, were not available across the included studies. In addition, the assessment of diastolic function in children is limited and can be unreliable due to poor interobserver agreement and the influence of the volumetric status or valve capacity. Finally, the number of included studies and the sample size were small, leading to substantial heterogeneity. There is still a paucity of available publications on longitudinal outcomes in MIS-C. Further studies with large cohorts of MIS-C and longer follow-up periods will help understanding long-term outcomes of MIS-C to establish optimal treatment strategies.
Conclusions
Our meta-analysis demonstrated largely favorable mid-term cardiovascular outcomes, including low mortality rate and normalization of LV systolic dysfunction in the majority of MIS-C patients. However, there were persistent coronary abnormalities and MR in a subset of patients at 6 months follow-up. Further studies with systematic long-term follow-up data in larger cohorts of MIS-C patients are warranted to validate our findings.
Data Availability
Access to the full study protocol and study data can be requested from the corresponding author.
References
Yasuhara J, Kuno T, Takagi H, Sumitomo N (2020) Clinical characteristics of COVID-19 in children: a systematic review. Pediatr Pulmonol 55:2565–2575
Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, Ramnarayan P, Fraisse A, Miller O, Davies P, Kucera F, Brierley J, McDougall M, Carter M, Tremoulet A, Shimizu C, Herberg J, Burns JC, Lyall H, Levin M (2020) Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 324:259–269
Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P (2020) Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395:1607–1608
Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, Barranco MA, Maxted AM, Rosenberg ES, Easton D, Udo T, Kumar J, Pulver W, Smith L, Hutton B, Blog D, Zucker H (2020) Multisystem inflammatory syndrome in children in New York state. N Engl J Med 383:347–358
Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, Singh AR, Li S, Tarquinio KM, Jaggi P, Oster ME, Zackai SP, Gillen J, Ratner AJ, Walsh RF, Fitzgerald JC, Keenaghan MA, Alharash H, Doymaz S, Clouser KN, Giuliano JS Jr, Gupta A, Parker RM, Maddux AB, Havalad V, Ramsingh S, Bukulmez H, Bradford TT, Smith LS, Tenforde MW, Carroll CL, Riggs BJ, Gertz SJ, Daube A, Lansell A, Coronado Munoz A, Hobbs CV, Marohn KL, Halasa NB, Patel MM, Randolph AG (2020) Multisystem inflammatory syndrome in US. children and adolescents. N Engl J Med 383:334–346
Cheung EW, Zachariah P, Gorelik M, Boneparth A, Kernie SG, Orange JS, Milner JD (2020) Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA 24:294–296
Dionne A, Mah DY, Son MBF, Lee PY, Henderson L, Baker AL, de Ferranti SD, Fulton DR, Newburger JW, Friedman KG (2020) Atrioventricular block in children with multisystem inflammatory syndrome. Pediatrics. https://doi.org/10.1542/peds.2020-009704
Theocharis P, Wong J, Pushparajah K, Mathur SK, Simpson JM, Pascall E, Cleary A, Stewart K, Adhvaryu K, Savis A, Kabir SR, Uy MP, Heard H, Peacock K, Miller O (2021) Multimodality cardiac evaluation in children and young adults with multisystem inflammation associated with COVID-19. Eur Heart J Cardiovasc Imaging 22:896–903
Valverde I, Singh Y, Sanchez-de-Toledo J, Theocharis P, Chikermane A, Di Filippo S, Kuciñska B, Mannarino S, Tamariz-Martel A, Gutierrez-Larraya F, Soda G, Vandekerckhove K, Gonzalez-Barlatay F, McMahon CJ, Marcora S, Napoleone CP, Duong P, Tuo G, Deri A, Nepali G, Ilina M, Ciliberti P, Miller O (2021) Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation 143:21–32
Yasuhara J, Watanabe K, Takagi H, Sumitomo N, Kuno T (2021) COVID-19 and multisystem inflammatory syndrome in children: a systematic review and meta-analysis. Pediatr Pulmonol 56(5):837–848
Capone CA, Subramony A, Sweberg T, Schneider J, Shah S, Rubin L, Schleien C, Epstein S, Johnson JC, Kessel A, Misra N, Mitchell E, Palumbo N, Rajan S, Rocker J, Williamson K, Davidson KW (2020) Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS-C) associated with SARS-CoV-2 infection. J Pediatr 224:141–145
Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, Soma VL, Maddux AB, Mourani PM, Bowens C, Maamari M, Hall MW, Riggs BJ, Giuliano JS Jr, Singh AR, Li S, Kong M, Schuster JE, McLaughlin GE, Schwartz SP, Walker TC, Loftis LL, Hobbs CV, Halasa NB, Doymaz S, Babbitt CJ, Hume JR, Gertz SJ, Irby K, Clouser KN, Cvijanovich NZ, Bradford TT, Smith LS, Heidemann SM, Zackai SP, Wellnitz K, Nofziger RA, Horwitz SM, Carroll RW, Rowan CM, Tarquinio KM, Mack EH, Fitzgerald JC, Coates BM, Jackson AM, Young CC, Son MBF, Patel MM, Newburger JW, Randolph AG, Overcoming C-I (2021) Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 325:1074–1087
Matsubara D, Kauffman HL, Wang Y, Calderon-Anyosa R, Nadaraj S, Elias MD, White TJ, Torowicz DL, Yubbu P, Giglia TM, Hogarty AN, Rossano JW, Quartermain MD, Banerjee A (2020) Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol 76(17):1947–1961
Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, Wacker J, Ovaert C, Hascoet S, Selegny M, Malekzadeh-Milani S, Maltret A, Bosser G, Giroux N, Bonnemains L, Bordet J, Di Filippo S, Mauran P, Falcon-Eicher S, Thambo JB, Lefort B, Moceri P, Houyel L, Renolleau S, Bonnet D (2020) Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 142(5):429–436
Penner J, Abdel-Mannan O, Grant K, Maillard S, Kucera F, Hassell J, Eyre M, Berger Z, Hacohen Y, Moshal K (2021) 6-month multidisciplinary follow-up and outcomes of patients with paediatric inflammatory multisystem syndrome (PIMS-TS) at a UK tertiary paediatric hospital: a retrospective cohort study. Lancet Child Adolesc Health 5:473–482
Farooqi KM, Chan A, Weller RJ, Mi J, Jiang P, Abrahams E, Ferris A, Krishnan US, Pasumarti N, Suh S, Shah AM, DiLorenzo MP, Zachariah P, Milner JD, Rosenzweig EB, Gorelik M, Anderson BR (2021) Longitudinal outcomes for multisystem inflammatory syndrome in children. Pediatrics. https://doi.org/10.1542/peds.2021-051155
Davies P, du Pré P, Lillie J, Kanthimathinathan HK (2021) One-year outcomes of critical care patients post-COVID-19 multisystem inflammatory syndrome in children. JAMA Pediatr 175:1281–1283
Capone CA, Misra N, Ganigara M, Epstein S, Rajan S, Acharya SS, Hayes DA, Kearney MB, Romano A, Friedman RA, Blaufox AD, Cooper R, Schleien C, Mitchell E (2021) Six month follow-up of patients with multi-system inflammatory syndrome in children. Pediatrics. https://doi.org/10.1542/peds.2021-050973
Barris DM, Keelan J, Ahluwalia N, Jhaveri S, Cohen J, Stern K, Seiden HS, Glass L (2022) Midterm outcomes and cardiac magnetic resonance imaging following multisystem inflammatory syndrome in children. J Pediatr 241:237-241.e231
Kucera F, Laurence C, Simmonds J, Gavela J, Bodnar T, Brogan P, Hoskote A, Skellett S, Moshal K, Bamford A, Khambadkone S (2021) Cardiac outcomes in severe acute respiratory syndrome coronavirus-2-associated multisystem inflammatory syndrome at a tertiary paediatric hospital. Cardiol Young 32(10):1585–1591
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1-34
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Research Ed) 372:n71
Centers for Disease Control and Prevention. (2020) Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19).
Royal College of Paediatrics and Child Health. (2020) Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS) – guidance for clinicians.
McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH, Saji TT, Pahl E (2017) Diagnosis, treatment, and long-term management of kawasaki disease: a scientific statement for health professionals from the american heart association. Circulation 135:e927–e999
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Matsubara D, Chang J, Kauffman HL, Wang Y, Nadaraj S, Patel C, Paridon SM, Fogel MA, Quartermain MD, Banerjee A (2022) Longitudinal assessment of cardiac outcomes of multisystem inflammatory syndrome in children associated with COVID-19 infections. J Am Heart Assoc 11:e023251
Mitchell EC, Romano A, Capone CA, Cooper R, Epstein S, Hayes DA, Parness IA, Schleien C, Misra N (2022) Multisystem inflammatory syndrome in children: salient echocardiogram findings in the acute phase and longitudinal follow-up. Prog Pediatr Cardiol. https://doi.org/10.1016/j.ppedcard.2022.101492
Awasthi P, Kumar V, Naganur S, Nallasamy K, Angurana SK, Bansal A, Manoj RK, Jayashree M (2022) Multisystem inflammatory syndrome in children: follow-up of a Cohort from North India. Am J Trop Med Hyg 106:1108–1112
Dove ML, Oster ME, Hashemi S, Slesnick TC (2022) Cardiac magnetic resonance findings after multisystem inflammatory syndrome in children. J Pediatr: https://doi.org/10.1016/j.jpeds.2022.02.049
Aziz OA, Sadiq M, Qureshi AU, Hyder N, Kazmi U, Batool A, Naz S, Mushtaq A, Bari A, Rashid J (2022) Short to midterm follow-up of multi-system inflammatory syndrome in children with special reference to cardiac involvement. Cardiol Young: https://doi.org/10.1017/S1047951122000828
Sanil Y, Misra A, Safa R, Blake JM, Eddine AC, Balakrishnan P, Garcia RU, Taylor R, Dentel JN, Ang J, Cashen K, Heidemann SM, Bauerfield C, Sethuraman U, Farooqi A, Aggarwal S, Singh G (2021) Echocardiographic indicators associated with adverse clinical course and cardiac sequelae in multisystem inflammatory syndrome in children with coronavirus disease 2019. J Am Soc Echocardiogr. https://doi.org/10.1016/j.echo.2021.04.018
Rowley AH (2020) Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat Rev Immunol 20:453–454
Jhaveri S, Ahluwalia N, Kaushik S, Trachtman R, Kowalsky S, Aydin S, Stern K (2021) Longitudinal echocardiographic assessment of coronary arteries and left ventricular function following multisystem inflammatory syndrome in children. J Pediatr 228:290-293.e291
Blondiaux E, Parisot P, Redheuil A, Tzaroukian L, Levy Y, Sileo C, Schnuriger A, Lorrot M, Guedj R, Ducou le Pointe H (2020) Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series. Radiology 297(3):E283–E288
Sengelov M, Jorgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, Nochioka K, Biering-Sorensen T (2015) Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging 8:1351–1359
Krupickova S, Bautista-Rodriguez C, Hatipoglu S, Kang H, Fraisse A, Di Salvo G, Piccinelli E, Rowlinson G, Lane M, Altamar Bermejo I, Moscatelli S, Wage R, Mohiaddin R, Pennell DJ, Voges I (2022) Myocardial deformation assessed by CMR in children after multisystem inflammatory syndrome (MIS-C). Int J Cardiol 346:105–106
Law YM, Lal AK, Chen S, Čiháková D, Cooper LT Jr, Deshpande S, Godown J, Grosse-Wortmann L, Robinson JD, Towbin JA (2021) Diagnosis and management of myocarditis in children: a scientific statement from the american heart association. Circulation 144:e123–e135
Shaykh R, Leftin S, Suh S, Spencer R, Gorelik M, Wilson PT, Diamond R (2021) Reversible coronary artery aneurysm with delayed anti-inflammatory therapy in multisystem inflammatory syndrome in children. JACC Case Rep 3:550–554
Villacis-Nunez DS, Hashemi S, Nelson MC, Flanagan E, Thakral A, Rodriguez F 3rd, Jaggi P, Oster ME, Prahalad S, Rouster-Stevens KA (2021) Giant coronary aneurysms in multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. JACC Case Rep 3:1499–1508
Bautista-Rodriguez C, Sanchez-de-Toledo J, Clark BC, Herberg J, Bajolle F, Randanne PC, Salas-Mera D, Foldvari S, Chowdhury D, Munoz R, Bianco F, Singh Y, Levin M, Bonnet D, Fraisse A (2021) Multisystem inflammatory syndrome in children: an international survey. Pediatrics. https://doi.org/10.1542/peds.2020-024554
Hasan MR, Al Zubaidi K, Diab K, Hejazi Y, Bout-Tabaku S, Al-Adba B, Al Maslamani E, Janahi M, Roscoe D, Lopez AP, Tang P (2021) COVID-19 related multisystem inflammatory syndrome in children (MIS-C): a case series from a tertiary care pediatric hospital in Qatar. BMC Pediatr 21:267
Elias MD, McCrindle BW, Larios G, Choueiter NF, Dahdah N, Harahsheh AS, Jain S, Manlhiot C, Portman MA, Raghuveer G, Giglia TM, Dionne A (2020) Management of multisystem inflammatory syndrome in children associated with COVID-19: a survey from the international Kawasaki disease registry. CJC Open 2:632–640
Ogata S, Tremoulet AH, Sato Y, Ueda K, Shimizu C, Sun X, Jain S, Silverstein L, Baker AL, Tanaka N, Ogihara Y, Ikehara S, Takatsuki S, Sakamoto N, Kobayashi T, Fuse S, Matsubara T, Ishii M, Saji T, Newburger JW, Burns JC (2013) Coronary artery outcomes among children with Kawasaki disease in the United States and Japan. Int J Cardiol 168:3825–3828
Friedman KG, Gauvreau K, Hamaoka-Okamoto A, Tang A, Berry E, Tremoulet AH, Mahavadi VS, Baker A, deFerranti SD, Fulton DR, Burns JC, Newburger JW (2016) Coronary artery aneurysms in Kawasaki Disease: risk factors for progressive disease and adverse cardiac events in the US population. J Am Heart Assoc. https://doi.org/10.1161/JAHA.116.003289
Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, Kazue T, Eto G, Yamakawa R (1996) Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 94:1379–1385
Dionne A, Newburger JW (2021) The electrocardiogram in multisystem inflammatory syndrome in children: mind your Ps and Qs. J Pediatr 234:10–11
Regan W, O’Byrne L, Stewart K, Miller O, Pushparajah K, Theocharis P, Wong J, Rosenthal E (2021) Electrocardiographic changes in children with multisystem inflammation associated with COVID-19: associated with Coronavirus disease 2019. J Pediatr 234:27-32.e22
Choi NH, Fremed M, Starc T, Weller R, Cheung E, Ferris A, Silver ES, Liberman L (2020) MIS-C and cardiac conduction abnormalities. Pediatrics. https://doi.org/10.1542/peds.2020-009738
Hallberg TC, Bjorklund AR, Slusher TM, Rodgers N (2021) Sinus bradycardia in a toddler with multisystem inflammatory syndrome in children (MIS-C) related to COVID-19. BMJ Case Rep. https://doi.org/10.1136/bcr-2021-242058
Tomlinson LG, Cohen MI, Levorson RE, Tzeng MB (2021) COVID-19-associated multisystem inflammatory syndrome in children presenting uniquely with sinus node dysfunction in the setting of shock. Cardiol Young 31:1202–1204
Clark BC, Sanchez-de-Toledo J, Bautista-Rodriguez C, Choueiter N, Lara D, Kang H, Mohsin S, Fraisse A, Cesar S, Sattar Shaikh A, Escobar-Diaz MC, Hsu DT, Randanne PC, Aslam N, Kleinmahon J, Lamour JM, Johnson JN, Sarquella-Brugada G, Chowdhury D (2020) Cardiac abnormalities seen in pediatric patients during the SARS-CoV2 pandemic: an international experience. J Am Heart Assoc 9:e018007
Tseng YS, Herron C, Garcia R, Cashen K (2021) Sustained ventricular tachycardia in a paediatric patient with acute COVID-19 myocarditis. Cardiol Young 31:1510–1512
Ouldali N, Toubiana J, Antona D, Javouhey E, Madhi F, Lorrot M, Léger PL, Galeotti C, Claude C, Wiedemann A, Lachaume N, Ovaert C, Dumortier M, Kahn JE, Mandelcwajg A, Percheron L, Biot B, Bordet J, Girardin ML, Yang DD, Grimaud M, Oualha M, Allali S, Bajolle F, Beyler C, Meinzer U, Levy M, Paulet AM, Levy C, Cohen R, Belot A, Angoulvant F (2021) Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA 325:855–864
Son MBF, Murray N, Friedman K, Young CC, Newhams MM, Feldstein LR, Loftis LL, Tarquinio KM, Singh AR, Heidemann SM, Soma VL, Riggs BJ, Fitzgerald JC, Kong M, Doymaz S, Giuliano JS Jr, Keenaghan MA, Hume JR, Hobbs CV, Schuster JE, Clouser KN, Hall MW, Smith LS, Horwitz SM, Schwartz SP, Irby K, Bradford TT, Maddux AB, Babbitt CJ, Rowan CM, McLaughlin GE, Yager PH, Maamari M, Mack EH, Carroll CL, Montgomery VL, Halasa NB, Cvijanovich NZ, Coates BM, Rose CE, Newburger JW, Patel MM, Randolph AG (2021) Multisystem inflammatory syndrome in children—initial therapy and outcomes. N Engl J Med 385:23–34
McArdle AJ, Vito O, Patel H, Seaby EG, Shah P, Wilson C, Broderick C, Nijman R, Tremoulet AH, Munblit D, Ulloa-Gutierrez R, Carter MJ, De T, Hoggart C, Whittaker E, Herberg JA, Kaforou M, Cunnington AJ, Levin M (2021) Treatment of multisystem inflammatory syndrome in children. N Engl J Med 385:11–22
Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, Behrens EM, Ferris A, Kernan KF, Schulert GS, Seo P, Son MBF, Tremoulet AH, Yeung RSM, Mudano AS, Turner AS, Karp DR, Mehta JJ (2021) American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 2. Arthritis Rheumatol 73:e13–e29
Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, Behrens EM, Kernan KF, Schulert GS, Seo P, Son MBF, Tremoulet AH, VanderPluym C, Yeung RSM, Mudano AS, Turner AS, Karp DR, Mehta JJ (2022) American college of rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 3. Arthritis Rheumatol 74(4):e1–e20
Harwood R, Allin B, Jones CE, Whittaker E, Ramnarayan P, Ramanan AV, Kaleem M, Tulloh R, Peters MJ, Almond S, Davis PJ, Levin M, Tometzki A, Faust SN, Knight M, Kenny S (2021) A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health 5:133–141
Chiotos K, Hayes M, Kimberlin DW, Jones SB, James SH, Pinninti SG, Yarbrough A, Abzug MJ, MacBrayne CE, Soma VL, Dulek DE, Vora SB, Waghmare A, Wolf J, Olivero R, Grapentine S, Wattier RL, Bio L, Cross SJ, Dillman NO, Downes KJ, Oliveira CR, Timberlake K, Young J, Orscheln RC, Tamma PD, Schwenk HT, Zachariah P, Aldrich ML, Goldman DL, Groves HE, Rajapakse NS, Lamb GS, Tribble AC, Hersh AL, Thorell EA, Denison MR, Ratner AJ, Newland JG, Nakamura MM (2021) Multicenter interim guidance on use of antivirals for children with Coronavirus disease 2019/severe acute respiratory syndrome coronavirus 2. J Pediatric Infect Dis Soc 10:34–48
Bansal N, Azeka E, Neunert C, Kim JS, Murray J, May L, Kirk C, Lorts A, Rosenthal D, VanderPluym C (2021) Multisystem inflammatory syndrome associated with COVID-19 anti-thrombosis guideline of care for children by action. Pediatr Cardiol 42:1635–1639
Acknowledgements
Not applicable.
Funding
The authors did not receive funding from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
Conceptualization: JY, TK, Writing—original draft preparation: JY, KM, Writing—review and editing: KW, TS, HT, NS, SL, TK, Data collection: JY, KM, Data analysis and interpretation: JY, KM, HT, TK, Supervision: NS, SL, TK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yasuhara, J., Masuda, K., Watanabe, K. et al. Longitudinal Cardiac Outcomes of Multisystem Inflammatory Syndrome in Children: A Systematic Review and Meta-Analysis. Pediatr Cardiol 44, 892–907 (2023). https://doi.org/10.1007/s00246-022-03052-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-022-03052-2