Abstract
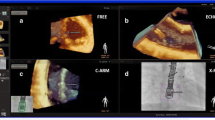
Cardiac 3D printing is mainly performed from magnetic resonance imaging (MRI) and computed tomography (CT) 3D datasets, though anatomic detail of atrioventricular (AV) valves may be limited. 3D echo provides excellent visualization of AV valves. Thus, we tested the feasibility and accuracy of 3D printing from 3D echo in this pilot series of subjects with congenital heart disease (CHD), with a focus on valve anatomy. Five subjects with CHD were identified. 3D echo data were converted to 3D printable files and printed in collaboration with 3D Systems Healthcare (Golden, Colorado). A novel technique for valve modeling was utilized using commercially available software. Two readers (KM, SA) independently measured valve structures from 3D models and compared to source echo images. 3D printing was feasible for all cases. Table 1 shows measurements comparing 2D echo to 3D models. Bland Altman analysis showed close agreement and no significant bias between 2D and digital 3D models (mean difference 0.0, 95% CI 1.1 to − 1.1) or 2D vs printed 3D models, though with wider limits of agreement (mean difference − 0.3, 95% CI 1.9 to − 2.6). Accuracy of 3D models compared to 2D was within < 0.5 mm. This pilot study shows 3D echo datasets can be used to reliably print AV and semilunar valve structures in CHD. The 3D models are highly accurate compared to the source echo images. This is a novel and value-added technique that adds incremental information on cardiac anatomy over current methods.









Similar content being viewed by others
Abbreviations
- 3D:
-
Three-dimensional
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- AV:
-
Atrioventricular
- CHD:
-
Congenital heart disease
- DICOM:
-
Digital imaging and communications in medicine
- CAD:
-
Computer-aided design
References
Sodian R, Weber S, Markert M, Loeff M, Lueth T, Weis FC et al (2008) Pediatric cardiac transplantation: three-dimensional printing of anatomic models for surgical planning of heart transplantation in patients with univentricular heart. J Thorac Cardiovasc Surg 136(4):1098–1099
Olivieri LJ, Krieger A, Loke Y-H, Nath DS, Kim PCW, Sable CA (2015) Three-dimensional printing of intracardiac defects from three-dimensional echocardiographic images: feasibility and relative accuracy. JASE 28(4):392–397
Costello JP, Olivieri LJ, Su L, Krieger A, Alfares F, Thabit O et al (2015) Incorporating three-dimensional printing into a simulation-based congenital heart disease and critical care training curriculum for resident physicians. Congenit Heart Dis 10(2):185–190
Farooqi KM, Uppu SC, Nguyen K, Srivastava S, Ko HH, Choueiter N et al (2015) Application of virtual three-dimensional models for simultaneous visualization of intracardiac anatomic relationships in double outlet right ventricle. Pediatr Cardiol. 37(1):90–98
Farooqi KM, Saeed O, Zaidi A, Sanz J, Nielsen JC, Hsu DT et al (2016) 3D Printing to guide ventricular assist device placement in adults with congenital heart disease and heart failure. JACC Heart Fail 4(4):301–311
Yoo S-J, Thabit O, Kim EK, Ide H, Yim D, Dragulescu A et al (2016) 3D printing in medicine of congenital heart diseases. 3D Print Med 2(1):1–12
Anwar S, Singh GK, Varughese J, Nguyen H, Billadello JJ, Sheybani EF et al (2017) 3D printing in complex congenital heart disease: across a spectrum of age, pathology, and imaging techniques. JACC Cardiovasc Imaging 10(8):953–956
Vukicevic M, Mosadegh B, Min JK, Little SH (2017) Cardiac 3D printing and its future directions. JACC Cardiovasc Imaging 10(2):171–184
Anwar S, Singh GK, Petrucci O, Eghtesady P, Woodard PK, Billadello JJ (2017) Adult congenital heart disease. In: Farooqi KM (ed) Rapid prototyping in cardiac disease. Springer, New York, pp 99–109
Yoo S-J, Spray T, Austin EH, Yun T-J, Van Arsdell GS (2017) Hands-on surgical training of congenital heart surgery using 3-dimensional print models. J Thorac Cardiovasc Surg 153(6):1530–1540
Anwar S, Singh GK, Miller J, Sharma M, Manning P, Billadello JJ et al (2018) 3D printing is a transformative technology in congenital heart disease. JACC Basic Transl Sci 3(2):294–312
Writing Committee Members, Hirshfeld JW, Ferrari VA, Bengel FM, Bergersen L, Chambers CE et al (2018) 2018 ACC/HRS/NASCI/SCAI/SCCT expert consensus document on optimal use of ionizing radiation in cardiovascular imaging: best practices for safety and effectiveness: a report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol 71(24):e283–e351
Babu-Narayan SV, Giannakoulas G, Valente AM, Li W, Gatzoulis MA (2016) Imaging of congenital heart disease in adults. Eur Heart J 37(15):1182–1195
Valente AM, Cook S, Festa P, Ko HH, Krishnamurthy R, Taylor AM et al (2014) Multimodality imaging guidelines for patients with repaired tetralogy of fallot: a report from the American Society of Echocardiography: developed in collaboration with the Society for Cardiovascular Magnetic Resonance and the Society for Pediatric Radiology. J Am Soc Echocardiogr 27(2):111–141
Prakash A, Powell AJ, Geva T (2010) Multimodality noninvasive imaging for assessment of congenital heart disease. Circul Cardiovasc Imaging 3(1):112–125
Mashari A, Montealegre-Gallegos M, Knio Z, Yeh L, Jeganathan J, Matyal R et al (2016) Making three-dimensional echocardiography more tangible: a workflow for three-dimensional printing with echocardiographic data. Echo Res Pract 3(4):R57–R64
Mahmood F, Owais K, Taylor C, Montealegre-Gallegos M, Manning W, Matyal R et al (2015) Three-dimensional printing of mitral valve using echocardiographic data. JACC Cardiovasc Imaging 8(2):227–229
Kapur KK, Garg N (2014) Echocardiography derived three-dimensional printing of normal and abnormal mitral annuli. Ann Card Anaesth 17(4):283–284
Witschey WRT, Pouch AM, McGarvey JR, Ikeuchi K, Contijoch F, Levack MM et al (2014) Three-dimensional ultrasound-derived physical mitral valve modeling. Ann Thorac Surg 98(2):691–694
Muraru D, Veronesi F, Maddalozzo A, Dequal D, Frajhof L, Rabischoffsky A et al (2017) 3D printing of normal and pathologic tricuspid valves from transthoracic 3D echocardiography data sets. Eur Heart J Cardiovasc Imaging 18(7):802–808
Premyodhin N, Mandair D, Ferng AS, Leach TS, Palsma RP, Albanna MZ et al (2018) 3D printed mitral valve models: affordable simulation for robotic mitral valve repair. Interact Cardiovasc Thorac Surg 26(1):71–76
Scanlan AB, Nguyen AV, Ilina A, Lasso A, Cripe L, Jegatheeswaran A et al (2018) Comparison of 3D echocardiogram-derived 3D printed valve models to molded models for simulated repair of pediatric atrioventricular valves. Pediatr Cardiol 39(3):538–547
Vukicevic M, Puperi DS, Jane Grande-Allen K, Little SH (2016) 3D printed modeling of the mitral valve for catheter-based structural interventions. Ann Biomed Eng 45(2):508–519
Harb SC, Rodriguez LL, Vukicevic M, Kapadia SR, Little SH (2019) Three-dimensional printing applications in percutaneous structural heart interventions. Circul Cardiovasc Imaging 12(10):e009014
Naoum C, Blanke P, Cavalcante JL, Leipsic J (2017) Cardiac computed tomography and magnetic resonance imaging in the evaluation of mitral and tricuspid valve disease: implications for transcatheter interventions. Circul Cardiovasc Imaging 10(3):TC06
Rajiah P, Moore A, Saboo S, Goerne H, Ranganath P, MacNamara J et al (2019) Multimodality imaging of complications of cardiac valve surgeries. RadioGraphics 39(4):932–956
Wunderlich NC, Beigel R, Ho SY, Nietlispach F, Cheng R, Agricola E et al (2018) Imaging for mitral interventions: methods and efficacy. JACC Cardiovasc Imaging 11(6):872–901
Cohen MS, Eidem BW, Cetta F, Fogel MA, Frommelt PC, Ganame J et al (2016) Multimodality imaging guidelines of patients with transposition of the great arteries: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance and the Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 29:571–621
Acknowledgement
We wish to thank the cardiac sonographers at the Pediatric Heart Center in Washington University in St. Louis who contributed to acquisitions of these 3D echo datasets. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Segmentation, model design, and 3D printing for cases in this paper were performed at no cost in collaboration with 3D Systems Healthcare (Golden, Colorado). Joe Fullerton, co-author, is an employee of 3D Systems. Shafkat Anwar: Consultant and shareholder – Printerprezz, Inc., a medical 3D printing start-up in Fremont, CA. No relevant conflict of interest related to this paper. All other authors have no relevant relationships or conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file 1 Supplemental Video 1: 2-month-old male infant (7 kg) with an unrepaired complete and balanced atrio-ventricular septal defect (AVSD) (MP4 2139 kb)
Supplementary file 2 Supplemental Video 2: 10-year-old (35 kg) female with severe aortic insufficiency due to prolapse of the non-coronary cusp and central lack of coaptation in the setting of a small, restrictive paramembranous VSD (MP4 2233 kb)
Appendix 1: Detailed Methodology: Image Segmentation, Post-processing and Three-Dimensional Printing
Appendix 1: Detailed Methodology: Image Segmentation, Post-processing and Three-Dimensional Printing
Once the five (5) patient studies were identified for modeling, DICOM data were anonymized and exported from the ultrasound device in a grayscale format with a Cartesian coordinate system, which allowed for importation of the volume into the segmentation software, Mimics v20.0 (Materialise, Leuven, Belgium). The DICOM files were uploaded to a secure remote server for transfer to collaborators at 3D Systems Healthcare for segmentation, model design, and 3D printing (JF, SA).
First, a frame was chosen for each study that best showed the pathology of interest in the systolic or diastolic phase. That single frame was imported into Mimics for segmentation. A combination of techniques was used to segment the anatomy, which involves creating masks that overlay the voxels of the imaging for each required anatomical component, including the endocardial surface, valve leaflets, and chordal structures. Each anatomical segmentation began with a threshold operation to select a range of gray values that best matched the anatomy. To segment the endocardial surface, a threshold was chosen in the darker range of gray values in the blood pool. To segment the valve leaflets, papillary muscles, and associated chordal structures, the chosen threshold range masked the higher intensity voxels of tissue. For both tissue and blood pool segmentations, the multiple slice edit tool was used extensively to fill gaps and eliminate extraneous voxels based on clinician interpretation (SA). This manual segmentation was required to overcome the low signal-to-noise ratio of 3D echocardiography, which makes thresholding alone insufficient to capture a continuous surface for modeling. In the final masks of the blood pool, leaflets and chordal structures were also masked to ensure that only the tissue masks would be used to define these fine structures, which allowed for a greater degree of control in creating the 3D form of these small structures. During this stage of segmentation, real-time web conferencing made it possible to simultaneously utilize the disparate expertise of the segmentor (JF) and clinician (SA), with the clinician providing guidance to the segmentor operating the software. Once segmentation was complete, the masks were exported in surface tessellation language (STL) format for further editing.
The STLs created from segmentation were then imported into Freeform Plus (3D Systems, Rock Hill, South Carolina, United States) to design the digital 3D models. A global smoothing was applied to each object to reduce the appearance of “stair steps” from voxels. To create hollow cardiac chambers, uniform positive offsets were applied to the negative space segmentations of the blood pool, followed by removing the original negative space from the offsets. Since the valve leaflets and chordal structures had been included in the negative space segmentations, they did not contribute to the topography of the endocardial surface created by this step of the design process, which allowed the designer to use the separately segmented leaflets and chordal structures to define the shape of those structures in the model. Since the valve leaflets are known to be thinner in vivo than they appear in the imaging data and resulting segmentation, it was desired to use the overall shape of the leaflets created by segmentation to design leaflets that would be thinner and uniform in thickness for easier comprehension of the shape and position. To that end, a series of 3D curves were plotted within the volume of the leaflets segmentation that described the valve annulus, free edge of the leaflets, and the surfaces of the leaflets. Then, the network of end-connected curves was converted to a continuous surface with the curves defining the boundaries, followed by adding a uniform thickness of one millimeter (1 mm) to the surfaces by expanding each side of the surface outward by 500 micrometers (500 µm). The final object was checked (SA) for conformance to the original segmented shape and then added to the hollow chambers. Remaining segmented chordal structures were then added. It was impractical to refine the rough segmented shapes of the chordal structures into thin, uniform shapes, so the original segmentation was used to create the final chordae tendinea in the models. As a result, they were depicted in the model as slightly thicker than in the source images, but it was a more reliable and repeatable way to visualize the locations and overall shapes of the chordal structures than trying to isolate individual chords from the mass of the segmentation. Finally, the separate anatomical components were assigned colors for identification purposes and exported from Freeform Plus in PLY format for digital visualization and 3D printing.
The physical models were 3D printed on a 3D Systems ProJet CJP 660Pro, which is a binder-jetting style of printer in which the print head deposits cyanoacrylate and ink onto a bed of mineral powder to bind together the powder particles. The printed models were removed from the powder bed of the printer and excess powder was removed with compressed air. Then the model was dipped in cyanoacrylate to infiltrate the surface of the model with additional binder, which hardens the model and brings out the colors of the ink. Finally, the models were dipped in wax as a protective layer against moisture and excess wax melted off in an oven.
Rights and permissions
About this article
Cite this article
Mowers, K.L., Fullerton, J.B., Hicks, D. et al. 3D Echocardiography Provides Highly Accurate 3D Printed Models in Congenital Heart Disease. Pediatr Cardiol 42, 131–141 (2021). https://doi.org/10.1007/s00246-020-02462-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-020-02462-4