Abstract
Extracorporeal cardiopulmonary resuscitation (ECPR) in children with cardiac arrest refractory to conventional cardiopulmonary resuscitation (CPR) has been reported with encouraging results. We reviewed outcomes of neonates with functional single ventricle (FSV) surviving post-cardiotomy ECPR after hospital discharge. Fifty-eight patients who required post-cardiotomy extracorporeal membrane oxygenation (ECMO) since the introduction of our ECPR protocol (January 2007–December 2011) were identified. Forty-one were neonates. Survival analysis was conducted. Of 41 neonates receiving post-cardiotomy ECMO, 32 had FSV. Twenty-one had ECPR. Fourteen underwent Norwood operation (NO) for hypoplastic left heart syndrome (HLHS). Seven had non-HLHS FSV. Four (of 7) underwent modified NO/DKS with systemic-to-pulmonary shunt (SPS), 2 SPS only and 1 SPS with anomalous pulmonary venous connection repair. Mean age was 6.8 ± 2.1 days. ECMO median duration was 7 days [interquartile range (IQR25–75: 4–18)]. Survival to ECMO discontinuation was 72% (15 of 21 patients) and at hospital discharge 62% (13 of 21 patients). The most common cause of late attrition was cardiac. At last follow-up (median: 22 months; IQR25–75: 3–36), 47% of patients were alive. Duration of ECMO and failure of lactate clearance within 24 h from ECMO deployment determined late survival after hospital discharge (p < 0.05). Rescue post-cardiotomy ECMO support in neonates with FSV carries significant late attrition. ECMO duration and failure in lactate clearance after deployment are associated with unfavorable outcome. Emphasis on CPR quality, refinement of management directives early during ECMO and aggressive early identification of patients requiring heart transplantation might improve late survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the first [1] reported use of extracorporeal membrane oxygenation (ECMO), its applications have expanded to include resuscitation after complex congenital heart disease (CHD) palliative or definitive correction in infants and children. There are no widely established criteria governing its use and results vary [2–5]. ELSO registry [6] and others [7] indicate that ECMO after repair of functional single ventricle (FSV) has poorer prognosis than other cardiac lesions.
Prolonged conventional cardiopulmonary resuscitation (CPR) is reportedly associated with extremely poor survival [2–6]. Increased duration of CPR in neonates and infants with hospital cardiac arrest carries substantial morbidity and high mortality [2–7]. Extracorporeal cardiopulmonary resuscitation (ECPR) is the rapid deployment of ECMO to provide immediate cardiovascular support for patients who have cardiac arrest refractory to conventional CPR strategies [3, 8]. The demonstrable survival benefit of ECPR over conventional CPR strategies has resulted in steadily increasing ECPR application. Appropriate patient selection and institutional effectiveness to deploy ECMO in a timely fashion may influence outcome [8–12].
Since 2007, we have encountered post-cardiotomy neonates with FSV of any type for which our established ECPR protocol was utilized. Neonates with FSV receiving post-cardiotomy ECPR were identified and late outcomes assessed.
Patients and Methods
Fifty-eight patients who required post-cardiotomy ECMO since the introduction of our ECPR protocol (January 2007–December 2011) were identified. From 41 neonates receiving post-cardiotomy ECMO, 32 had FSV. Twenty-three had ECPR. Two had a second ECMO run. Twenty-one with an index course of ECPR were included in the analysis.
Patients were included in the ECPR group if venoarterial ECMO was used as part of the initial active resuscitation from a cardiac arrest. Patients hemodynamically unstable but without active cardiac arrest were excluded. A retrospective review was conducted and survival analysis undertaken.
Vasoactive-inotropic score (VIS) was calculated as previously described [13]. VIS was classified as: (1) Class I: ≤ 10, (2) Class II: 11–14, (3) Class III: 15–19, (4) Class IV: 20–24 and (5) Class V: ≥ 25.
Systolic function was qualitatively evaluated by apical and parasternal short-axis images as (SVNL) normal shortening function (SF) without myocardial wall dysfunction, (RVNL-1) mild-to-moderate myocardial wall dysfunction with reduced SF (up to 50% of normal) and (SVNL-2) severe myocardial wall hypokinesis with severely reduced SF (by more than 50% of normal). Echocardiograms were obtained within 24 h from ECMO deployment, during ECMO, prior to decannulation, at hospital discharge and periodically thereafter. All echocardiograms were reviewed by an independent echocardiographer blinded to the study.
Significant adverse events were categorized as follows: (1) brain/neurological injury (BNI) (clinical or electroencephalographic seizures, significant central nervous system hemorrhage or ischemia, intraventricular hemorrhage (>grade I) based on ultrasound or computed tomographic scan); (2) renal injury (serum creatinine ≥1.7 mg/dL or need for dialysis); (3) sepsis; (4) respiratory complications (ventilator-associated pneumonia, acute respiratory distress syndrome, or pulmonary hemorrhage); (5) cardiac complications [new onset arrhythmias that required treatment, SVNL-2, heart transplantation (HTxP) or other major cardiac event]; (6) gastrointestinal complications (hemorrhage or ischemia); (7) bleeding (cannulation or surgical site, hemothorax and hemopericardium requiring intervention); (8) unplanned reoperation/reintervention (other than the anticipated staged palliation); and (9) multiple organ failure (MOF) (more than 2 major organ failures requiring medical intervention to maintain function).
ECMO decannulation was considered successful when “native” circulation was maintained for 48 h after decannulation without ECMO recannulation. Primary outcomes were survival to discharge from the hospital, late death (defined as death any time after hospital discharge) and “late attrition.” “Late attrition” was defined as the combined endpoint involving late death, need for HTxP or failure to reach suitability for Fontan completion (CF).
The study was approved by the institutional review board. Need for parental consent was waived.
Management Principles
ECMO rapid deployment strategy, ECPR protocols and intensive care unit management were described in detail previously [14]. Application of rapid ECMO deployment in FSV patients who experienced cardiac arrest intractable to standard CPR strategies followed certain principles. Indications and mode of deployment remained constant throughout the study. Once ECPR was required, the predefined protocol was initiated. Timing of weaning was dependent on hemodynamic stability during ECMO support, correction of the underlying cause and the presence of residual cardiac lesions. Echocardiography was frequently used to assess myocardial function during weaning process. Weaning and separation from ECMO assist were accomplished as previously described [14].
Statistical Analysis
Data are expressed as mean ± standard deviation (SD) or median with interquartile range (25–75 IQR) for continuous variables and as frequencies and percentages for categorical variables. Continuous variables were compared by using the Mann–Whitney and Student’s t tests, as appropriate. Fisher’s exact test and Chi-square analyses were used for dichotomous and categorical variables. The probability of freedom from events was estimated according to Kaplan–Meier method. For all endpoints, time was measured from initiation of ECMO. All non-mortality secondary endpoints were considered to have been censored in the event of late death, HTxP or determination of Fontan unsuitability. Univariate analysis was carried out using p value of <0.05. Due to small sample, multivariate logistic regression model was not performed. SPSS v.20 and R v.2.13.2 were used for all descriptive and inferential analyses. A 95% level of significance was set for all inferential tests.
Results
Patients Characteristics
From 41 neonates receiving post-cardiotomy ECMO, 32 had FSV. Twenty-one had an index course of ECPR. Fourteen underwent Norwood operation (NO) for hypoplastic left heart syndrome (HLHS). One had restrictive atrial septal defect prior to NO. Pulmonary blood flow reconstitution was established with right ventricle-to-pulmonary shunt (RVPAS) (n = 8) and modified Blalock–Taussig shunt (mBTS) (n = 6). Seven neonates had non-HLHS FSV. Four underwent Damus–Kaye–Stansel (DKS) or modified NO (2 RVPAS and 2 mBTS). Three (of 4) were diagnosed with double-outlet right ventricle, mitral atresia, aortic atresia (two with interrupted aortic arch) and one with double-inlet left ventricle, transposed great arteries, hypoplastic aortic valve and mitral atresia. Three neonates underwent systemic-to-pulmonary shunt (SPS). One had obstructed anomalous pulmonary venous connection.
Two patients had extracardiac anomalies associated with chromosomal or genetic abnormalities (GS/CA); one with Turner’s; and one with VACTERL syndrome.
Mean age and weight were 7.5 ± 2.7 days and 3.57 ± 1.7 kg, respectively. Three patients had gestational age <35 weeks at birth and 4 weighted <2.5 kg (LBW) at the time of surgical repair. Major indication for ECPR was acute cardiac arrest (17; 81%) and respiratory failure followed by cardiac arrest (4; 19%). The median interval between the beginning of CPR and the initiation of ECMO (CPR duration) was 36 min (IQR25–75: 25–52).
Demographic and clinical data for all patients treated with ECPR are depicted in Table 1.
Early Results and Hospital Survival
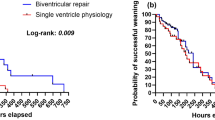
ECMO median duration was 7 days (IQR25–75: 4–21). ECMO was successfully discontinued in 15 (72%) patients. All 15 maintained “native” circulation for 48 h. Thirteen (62%) patients survived to hospital discharge (Fig. 1).
Longitudinal follow-up and outcome, (a)median: 22 month (IQR25–75: 3–36) BTS Blalock–Taussig shunt, DKS Damus–Kay–Stansel operation, FSV functional single ventricle, HLHS hypoplastic left heart syndrome, SPS systemic-to-pulmonary shunt, TAPVC total anomalous pulmonary venous connection, HTxP heart transplantation
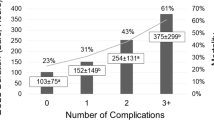
Non-survivors had overall significantly more complications than survivors. One or combinations of them were present in over 70% of non-survivors compared to 30% of survivors (p 0.05).
The causes for hospital mortality included multi-organ failure (MOFS) (n = 6, 28%), sepsis or necrotizing enterocolitis (NEC) (n = 5, 24%), intraventricular or cerebral hemorrhage (n = 4, 19%) and failure of cardiac recovery (n = 2, 9%). None suffered ischemic BNI as manifested by clinical or imaging findings. There was one reoperation during ECMO for severe tricuspid valve insufficiency (NO-RVPAS). The patient suffered major intraventricular hemorrhage, and ECMO support was withdrawn. Six more reoperations (two in the same patient) were performed off ECMO and prior to hospital discharge. One had shunt revision, one sternal deep wound infection and two peritoneal dialysis catheter insertion. One suffered from NEC and had bowel resection. Only one survived hospital discharge.
Both patients with GS/CA decannulated from ECMO but did not survive hospital discharge.
Seven patients during ECMO and three after decannulation required filtration or peritoneal dialysis. Four did not survive hospital discharge. Serum creatinine level and achieving negative fluid balance (within 72 h following ECMO and 24 h following decannulation from ECMO) were not statistically different between survivors and non-survivors (p 0.7). In 55% of non-survivors, negative fluid balance was achieved in more than 72 h following ECMO.
There was echocardiographic evidence of ventricular recovery (SVNL or SVNL-1) within 48 h following ECMO in 90% of survivors (vs 55% of non-survivors, p 0.08). More than 50% of those with SVNL-2 after 48 h following ECMO did not survive hospital discharge.
None received heart transplantation bridged from ECMO or prior to hospital discharge.
Early outcomes between ECPR survivors and non-survivors are summarized in Table 2.
Late Attrition and Time-Related Events
At last follow-up [median: 22 months; (IQR25–75: 3–36)], 47% of patients were alive and neurologically intact. Three patients died after hospital discharge at a mean interval of 5.7 ± 3.3 months. One died from NEC. Another died from acute shunt occlusion followed by cardiac arrest prior to arrival in the emergency room. The third had a ventricular fibrillation arrest in outside facility from which she succumbed.
After hospital discharge, 2 survivors required HTxP after Stage II palliation at 7.9 and 16.3 months, respectively, due to severe heart failure. Both are alive at last follow-up. One more deemed unsuitable for CF.
Freedom from late attrition at 3, 6, 18 and 36 months was 59.2 ± 12.9, 51.5 ± 14.7, 41.6 ± 15.1 and 34.3 ± 15.7%, respectively (Fig. 2). Attrition after hospital discharge exceeded 25% for the combined endpoint (death, HTxP or determination of CF unsuitability). Survival attenuation after hospital discharge approached 15%. The combined endpoint of attrition was achieved with an average rate of 0.71–0.75% and that of survival attenuation with 0.42–0.45% per month, respectively, for the time period until last follow-up.
One survivor suffered transient ischemic cerebral event following Stage II palliation. The patient recovered completely and is alive at last follow-up. Excluding planned Stage II and II or HTxP, 5 had unplanned reoperations after hospital discharge. One after NO-RVPAS had shunt revision prior to Stage II. Two had AV valve repair. One required surgery for post-ischemic bowel obstruction and one for PD catheter insertion. Four (of 5) were alive at last follow-up. The most common cause of late attrition was cardiac related. Late outcomes between survivors and non-survivors are summarized in Table 3.
Freedom from any significant event (different than the primary endpoints) after ECMO decannulation requiring readmission with the intent to treat (unplanned intervention/reoperation, end-organ adverse event) at 3, 6, 18 and 36 months was 58.5 ± 13.2, 49.3 ± 15.5, 42.1 ± 17.4 and 42.1 ± 17.4%, respectively (Fig. 3).
Risk Factors for Late Survival
Mean CPR duration between ECPR survivors and non-survivors was 37.2 ± 5.8 and 40.6 ± 6.9 min, respectively (p 0.09).
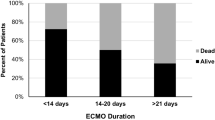
Median duration of ECMO between survivors and non-survivors was 4 days (IQR25–75: 3–7) and 9 days (IQR25–75: 6–13), respectively (p 0.01).
From 24 variables included, failure of serum lactate clearance within 24 h following rescue ECMO (p 0.02) and ECMO duration (p 0.01) were associated with attenuated survival after hospital discharge (Table 1). Cardiac-related adverse events remained a constant risk factor throughout the study.
Discussion
Despite increasing experience with ECMO support in children with cardiac failure, survival over more than decade has remained unchanged [2–6, 8, 15, 16].
Rescue ECMO is a potentially lifesaving intervention to reverse refractory cardiopulmonary arrest after surgical intervention for CHD. When ECMO is deployed during CPR, efforts outcomes vary and are, usually, poorer compared to non-rescue ECMO [4, 8, 17]. It is indicated [18] that neonates with FSV are more vulnerable to myocardial damage and less tolerant to any disturbance added to the demands of balancing two circulations. Thus, the most important factor for achieving favorable outcome with ECPR is the prompt establishment of adequate organ perfusion. It is our strategy to consider ECPR after cardiotomy as long as there is witnessed arrest with lack of recovery of “native” cardiac function within 1 min of CPR and absence of comorbid conditions that will preclude survival. Once the decision is made, our target deployment time is 25–45 min.
The intended focus was the subset of neonates with complex FSV (including HLHS). This was a follow-up of our original study [14] to further assess the attrition toll observed after hospital discharge. One of its unique features is that “late attrition” was considered not only the physical demise (death) of the patient, but also the failure in accomplishing long-term transition to total cavopulmonary connection (CF) or non-HTxP status. Analysis was undertaken between survivors and non-survivors.
We were able to demonstrate in-hospital survival over 60%. This is different from what has been reported by others with observed survival at hospital discharge between 34 and 52% [8, 15, 16, 18, 19] and is favorably compared to those with post-cardiotomy non-rescue ECMO [12, 15, 20, 21].
Late mortality among hospital survivors after ECMO ranges between 4 and 12% [22]. The reported causes of death relate mainly to the underlying heart condition and/or associated illnesses rather than ECMO support itself. Our study indicated that late attrition after hospital discharge exceeded 25% for the combined endpoint (death, HTxP or unsuitability for CF) with an average rate of 0.71–0.75% per month until last follow-up. At 3-year interval from ECMO decannulation, nearly two-thirds of studied patients were dead, required HTxP or deemed not suitable candidates for CF.
As previously reported [10, 14, 17], CPR duration prior to ECMO deployment is not associated with decreased hospital survival. This study expands this notion that duration of CPR does not influence late survival. CPR adequacy and potential link of ineffective CPR to poor outcome were not assessed.
Consistent with other studies [9, 12, 17, 20, 21, 23], ECMO duration was statistically different in the ECPR survivors compared to non-survivors at late follow-up. This indicates that longer duration of extracorporeal support carries constant complement of risks and has future deleterious effects on end-organ systems which may contribute to late attrition [24].
One consistent finding in other studies [11, 12, 20, 21] is that serum lactate abnormal values indicate either the overall hypoperfusion before or oxygen delivery/extraction mismatch following ECMO deployment. We previously demonstrated [14] that peak serum lactate level (threshold value 8.9 mmol/L) within 24 h following ECPR predicts unfavorable outcome. Furthermore, failure of serum lactate clearance within 24 h after ECMO deployment is associated with late mortality in this series. We strongly advocate delivering higher initial ECMO flows after intractable cardiac arrest in FSV when end organs including the myocardium have likely had a degree of hypoxic injury. However, persistent need for higher flows may represent ongoing myocardial dysfunction, residual defect or technically imperfect operative outcome. Persistent higher flows are likely to promote decreased lung compliance and plasma exchange, strong indicators of early mortality [23].
There was a clinical trend noted toward decreased survival in patients whose systemic ventricle continued underperforming after 48 h following ECMO. This did not reach statistical significance. We advocate that all hemodynamically significant residual lesions to be corrected prior to ECMO withdrawal, as this may impact not only successful transition to stable “native” circulation, but also, determine late cardiac performance. Planned echocardiographic evaluation helps to assess ventricular recovery, identify hemodynamically significant residual lesions, guide effective management and ECMO flows or determine the need for early HTxP. Attenuated myocardial recovery, despite prolonged ECMO support, calls for early consideration of alternative supportive (ventricular assist device) or replacement (HTxP) therapy. HTxP was reserved in 2 cases within 18 months from hospital discharge due to progressive deterioration of ventricular function. Its utility as a bridge from ECMO was not attempted.
Major complications are, rather, inherent phenomenon associated with ECMO [14, 22, 23]. Hemorrhagic events and increased transfusion requirements are reportedly associated with significant mortality and morbidity early after ECMO [20, 24]. Meticulous hemostasis, cell salvage, judiciously escalating anticoagulation following ECMO, use of polymethylpentene hollow-fiber oxygenator and miniaturization of ECMO circuit with heparin-bonded biocompatible surface helped eliminating major bleeding complications and, thus, reducing exposure to blood products as demonstrated here.
Renal dysfunction following ECMO represents a surrogate marker of organ perfusion, and it has been shown to influence morbidity [11, 14, 20, 21]. Renal morbidity might extend beyond the immediate post-ECMO period. Our findings indicate that despite the need for renal replacement therapy, renal dysfunction was not detrimental for late survival.
Children undergoing ECMO are vulnerable to neurodevelopmental disability (NDD). Acute BNI after ECPR in neonates and infants was reportedly present in more than one-third of survivors [6, 7, 25]. In ECMO survivors late after hospital discharge, there have been varied disability rates dependent on the study’s reported definition [23, 25–27]. The incidence of BNI did not statistically differ between late survivors and non-survivors in our study. Due to the small sample and relatively short follow-up period, no meaningful analysis of predictors was possible. History of CPR, perioperative seizures and brain imaging abnormalities or microcephaly are all significant risk factors for NDD in complex CHD post-ECMO survivors, and appropriate surveillance is recommended [28].
LBW and GS/CA along with FSV have reportedly associated with increased odds of unfavorable outcome [23, 27, 29]. Only one (of 4) with LBW and none (of 2) with GS/CA were alive at last follow-up. The influence of LBW or GS/CA on clinical outcome did not reach statistical significance between late survivors and non-survivors. This probably reflects in the small sample studied.
This series is subject to limitations of a single-site retrospectively ascertained patient cohort. Collection of variables was not under the control of investigator, and therefore, variables that could have had an important influence on outcome may not be available for analysis. Echocardiographic SV qualitative assessment carries inherent limitations, especially in dominant right ventricle. Combined qualitative and quantitative evaluation would have been preferred, but measurements were not available at the time of the study. There was no uniform appraisal of neurologic function by an independent pediatric neurology specialist. Statistically significant differences might be hampered by the small sample and duration of follow-up. Finally, the relatively limited power of the study precluded logistic regression analysis.
In conclusion, despite the heavy toll in resources required, post-cardiotomy ECMO for neonates with complex FSV and intractable cardiac arrest carries a favorable outcome for more than 60% of the patients at hospital discharge. ECMO duration and serum lactate clearance within 24 h following ECPR might influence late survival. Late attrition following hospital discharge exceeds a monthly rate of 0.7%. Cardiac-related events are the dominant cause for late attrition. Uncorrected residual lesions are associated with unfavorable outcome. An interdisciplinary structure and proficiency in ECMO deployment justify an aggressive strategy toward timely application of ECPR when no other morbid conditions that severely limit survival are present. Proper patient selection, emphasis on CPR quality, refinement of management directives early during ECMO and early identification of patients requiring heart transplantation might improve late survival.
References
Baffes TG, Fridman JL, Bicoff JP et al (1970) Extracorporeal circulation for support of palliative cardiac surgery in infants. Ann Thorac Surg 10:354–363
de Mos N, van Litsenburg RR, McCrindle B et al (2006) Pediatric in-intensive-care-unit cardiac arrest: incidence, survival, and predictive factors. Crit Care Med 34:1209–1215
Morris MC, Wernovsky G, Nadkarni VM (2004) Survival outcomes after extracorporeal cardiopulmonary resuscitation instituted during active chest compressions following refractory in-hospital pediatric cardiac arrest. Pediatr Crit Care Med 5:440–446
Thiagarajan RR, Laussen PC, Rycus PT et al (2007) Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation 116:1693–1700
Rhodes JF, Blaufox AD, Seiden HS et al (1999) Cardiac arrest in infants after congenital heart surgery. Circulation 100(19 Suppl):II194–II199
ECMO Registry of extracorporeal life support organization (ELSO). Ann Arbor (2002)
Chow G, Koirala B, Armstrong D et al (2004) Predictors of mortality and neurological morbidity in children undergoing extracorporeal life support for cardiac disease. Eur J Cardio-Thorac Surg 26:38–43
Jacobs JP, Ojito JW, McConaghey T et al (2000) Rapid cardiopulmonary support for children with complex congenital heart disease. Ann Thorac Surg 70:742–749
Aharon AS, Drinkwater DC Jr, Churchwell KB et al (2001) Extracorporeal membrane oxygenation in children after repair of congenital cardiac lesions. Ann Thorac Surg 72:2095–2101
Chen YS, Chao A, Yu HY et al (2003) Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. J Am Coll Cardiol 41:197–203
del Nido PJ, Dalton HJ, Thompson AE et al (1992) Extracorporeal membrane oxygenator rescue in children during cardiac arrest after cardiac surgery. Circulation 86(5 Suppl):II300–II304
Hoskote A, Bohn D, Gruenwald C et al (2006) Extracorporeal life support after staged palliation of a functional single ventricle: subsequent morbidity and survival. J Thorac Cardiovasc Surg 131:1114–1121
Gaies MG, Gurney JG, Yen AH et al (2010) Vasoactive-Inotropic score as predictor of mortality and morbidity in infants after cardiopulmonary bypass. Pediatr Crit Care Med 11(2):123–128
Polimenakos AC, Wojtyla P, Smit PJ et al (2011) Postcardiotomy extracorporeal cardiopulmonary resuscitation in neonates with complex single-ventricle: analysis of outcomes. Eur J Cardiothorac Surg 40:1396–1405
Alsoufi B, Al-Radi O, Gruenwald C et al (2009) Extracorporeal life support following cardiac surgery in children: Analysis of risk factors and survival in a single institution. Eur J Cardio-Thorac Surg 35:1004–1011
Hannan RL, Ojito JW, Ybarra MA et al (2006) Rapid cardiopulmonary support in children with heart disease. Ann Thorac Surg 82:1637–1641
Alsoufi B, Al-Radi O, Nazer RI et al (2007) Survival outcomes after rescue extracorporeal cardiopulmonary resuscitation in pediatric patients with refractory cardiac arrest. J Thorac Cardiovasc Surg 134:952–959
Pizarro C, DA Davis, Healy RM et al (2001) Is there a role for extracorporeal life support after stage I Norwood? Eur J Cardio-Thorac Surg 19:294–301
Raymond TT, Cunnyngham CB, Thompson MT et al (2010) Outcomes among neonates, infants and children after extracorporeal cardiopulmonary resuscitation for refractory in-hospital pediatric cardiac arrest. Pediatr Crit Care Med 11:362–371
Kumar S, Zurakowski D, Dalton H et al (2010) Extracorporeal membrane oxygenation in postcardiotomy patients: factors influencing outcome. J Thorac Cardiovasc Surg 140:330–336
Kolovos NS, Bratton SL, Moler FW et al (2003) Outcome of pediatric patients treated with extracorporeal life support after cardiac surgery. Ann Thorac Surg 76:1435–1442
Brown KL, Ichord R, Marino BS et al (2013) Outcomes following extracorporeal membrane oxygenation in children with cardiac disease. Pediatr Crit care Med 14:S73–S83
Chrysostomou C, Morell VO, Kuch BA et al (2013) Short and intermediate survival after extracorporeal membrane oxygenation in children with cardiac disease. J Thorac Cardiovasc Surg 146:317–325
Hadley JS, Wang JE, Michaels LC et al (2007) Alterations in inflammatory capacity and TLR expression on monocytes and neutrophils after cardiopulmonary bypass. Shock 27:466–473
Barrett CS, Bratton SL, Salvin JW et al (2009) Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med 10:445–451
Lequier L, Joffe AR, Robertson CM, Western Canadian Complex Pediatric Therapies Program Follow-up Group et al (2008) Two-year survival, mental, and motor outcomes after cardiac extracorporeal life support at less than five years of age. J Thorac Cardiovasc Surg 136:976–983
McMullan DM, Thiagarajan RR, Smith KM et al (2014) Extracorporeal cardiopulmonary resuscitation outcomes in term and premature neonates. Pediatr Crit Care Med 15:e9–e16
Marino BS, Lipkin PH, Newburger JW et al (2012) Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management. Circulation 216:1143–1172
Kane DA, Thiagarajan RR, Wypij D et al (2010) Rapid response extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in children with cardiac disease. Circulation 122(11 suppl):S241–S248
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of the article.
Informed Consent
The study was approved by the institutional review board. Need for parental consent was waived.
Rights and permissions
About this article
Cite this article
Polimenakos, A.C., Rizzo, V., El-Zein, C.F. et al. Post-cardiotomy Rescue Extracorporeal Cardiopulmonary Resuscitation in Neonates with Single Ventricle After Intractable Cardiac Arrest: Attrition After Hospital Discharge and Predictors of Outcome. Pediatr Cardiol 38, 314–323 (2017). https://doi.org/10.1007/s00246-016-1515-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-016-1515-3