Abstract
We hypothesized that postoperative sedation with dexmedetomidine/fentanyl would be effective in infants and neonates with congenital heart disease and pulmonary arterial hypertension (PAH). Children who were <36 months of age, had congenital heart disease with PAH, and had been treated at our hospital between October 2011 and April 2013 (n = 187) were included in this retrospective study. Either dexmedetomidine/fentanyl (Group Dex) or midazolam/fentanyl (Group Mid) was used for postoperative sedation. The main outcome variables included delirium scores, supplemental sedative/analgesic drugs, ventilator use, and sedation time. Baseline demographics and clinical characteristics were similar between the two groups. The Pediatric Anesthesia Emergence Delirium scale (5.2 ± 5.3 vs. 7.1 ± 5.2 in the Dex and Mid groups, respectively; P = 0.016) and the incidence of delirium (18.2 vs. 32.0 % in the Dex and Mid groups, respectively; P = 0.039) were significantly lower in the Dex group than in the Mid group. Total sufentanil, midazolam, and propofol doses given during the operation did not differ between the two groups. Group Dex patients required significantly lower doses of adjunctive sedative/analgesic drugs than group Mid patients in the cardiac intensive care unit (CICU; midazolam, P = 0.007; morphine, P < 0.001). In conclusion, we found no differences between dexmedetomidine/fentanyl and midazolam/fentanyl in terms of the duration of sedation, mechanical ventilator use, and CICU stay in children with PAH. However, patients in the Dex group required a lower additional sedative/analgesic drugs and had a lower incidence of delirium than patients in the Mid group.
Similar content being viewed by others
Introduction
Pre- and postoperative treatment of children with congenital heart disease with severe pulmonary arterial hypertension (PAH) poses enormous challenges in the intensive care unit (ICU), especially during the postoperative phase [6, 8]. The main triggers of pulmonary hypertension to avoid during postoperative treatment include hypoxemia, hypercapnia, metabolic acidosis, airway obstruction, and volume overload, which contribute to pulmonary arterial hypertensive crises induced by a sympathetic stress response. Therefore, deep sedation and analgesia are extremely important for the postoperative treatment of PAH in children.
Intravenous benzodiazepines and opioids are the most commonly used drugs for sedation and analgesia in the pediatric ICU (PICU) [12, 23]. Midazolam, lorazepam, and diazepam are the most commonly used benzodiapenes in the PICU [15]. Fentanyl is the most commonly used opioid for procedural sedation because of its pharmacokinetic profile, analgesic potency, and low cost. The combination of benzodiazepines and opioids often results in satisfactory sedation of children; however, they are associated with adverse reactions such as respiratory depression, prolonged mechanical ventilation, and prolonged ICU stay [7, 17].
Dexmedetomidine (Dex) is a selective alpha-2-adrenergic receptor agonist that possesses sedative, anxiolytic, and analgesic effects without causing significant respiratory depression [10]. Dex is used in children with heart disease undergoing MRI scans, cardiac catheterization examinations, and other diagnostic and treatment procedures [13, 14, 19]. Additionally, several studies [5, 9] have shown that Dex can provide adequate sedation for acutely ill children when administered for more than 24 h. Studies investigating the use of Dex in children are limited to small-scale randomized studies [13, 21, 22]. Therefore, we designed this study to compare the quality of sedation between midazolam/fentanyl and Dex/fentanyl in infants and children with congenital heart disease following cardiac surgery, and the primary endpoint included delirium scores and the use of rescue adjuvant drugs, whereas the second endpoint included the length of mechanical ventilator and sedation treatment.
Materials and Methods
Patients
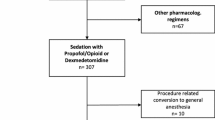
We performed a retrospective study of pediatric patients with congenital heart disease who had been operated on between October 2011 and April 2013. Children who were less than 36 months of age, had PAH (mean pulmonary arterial pressure, mPAP ≥ 25 mmHg), and received Dex/fentanyl or midazolam/fentanyl for sedation treatment were included in the study. Patients were diagnosed with PAH before surgery by estimating the speed of tricuspid regurgitation with echocardiography. Patients with incomplete medical records and withdrawal in the course of treatment were excluded from the study. This study was approved by the Ethics Committee of Chengdu Military General Hospital (201109).
Depending on the drug used for sedation, the patients were divided into two groups: Patients in group Dex had been administered dexmedetomidine/fentanyl, and group Mid patients had been administered midazolam/fentanyl.
Postoperative Sedation
In our department, continuous sedation and analgesia treatment were used for all pediatric patients with PAH after operation, fentanyl as an analgesic was used for all patients, and infusion of either Dex or midazolam was decided randomly by ICU physicians according to their own preferences. Continuous infusion of Dex/fentanyl (group Dex) or midazolam/fentanyl (group Mid) was often initiated in the immediate postoperative period when the children are brought to the CICU from the operation room. The infusion is often discontinued within 1 h of extubation from mechanical ventilation. The treatment doses were as follows: 0.25–0.75 µg kg−1 h−1 of Dex and 0.5–3 µg kg−1 min−1 of midazolam, and the maintenance dose of fentanyl was 1.0–1.5 µg kg−1 h−1. The quality of sedation was assessed every 2 h using the Ramsay sedation scale [22], and children received diazepam or midazolam when they were brought to the CICU postoperatively (0.1 mg kg−1 each time) if the Ramsay scores were lower than 3. Nurses adjusted the rate of continuous infusion based on standard procedures at our institution to achieve a Ramsay score between 3 and 5. If the Ramsay score could not be achieved even after the maximum maintenance dose was reached, the attending doctor administered diazepam or midazolam (0.1 mg kg−1 each time) or morphine (0.1 mg kg−1 each time) as adjunctive treatment.
Data Collection
The following data were collected: demographic information (age, weight, primary diagnosis, pulmonary artery pressure, and cardiopulmonary bypass time), mechanical ventilation information (ventilator time and reintubation), incidence of delirium, length of CICU and hospital stays, sedative and analgesic regimens in the operation room, and supplement sedative/analgesic drugs (diazepam, midazolam, or morphine) administered in the CICU. The mPAP was direct measurement using arterial sensor in the operation room by the surgeon. The baseline demographic data, Risk Adjustment in Congenital Heart Surgery (RACHS-1) categories [1], and the Pediatric Risk of Mortality III (PRISM III-24) scores [16] were recorded for all patients. The emergence delirium was evaluated by using Pediatric Anesthesia Emergence Delirium (PAED) scale score [20], and bedside nurses recorded the PAED scale starting from the time of awakening or whenever the child became agitated. The PAED scale includes five items: (1) The child makes eye contact with the caregiver, (2) the child’s actions are purposeful, (3) the child is aware of his/her surroundings, (4) the child is restless, and (5) the child is inconsolable. Items 1, 2, and 3 are reverse-scored as follows: 4, not at all; 3, just a little; 2, quite a bit; and 1, very much; 0, extremely. Items 4 and 5 are scored as follows: 0, not at all; 1, just a little; 2 quite a bit; 3, very much; and 4, extremely. The scores of each item were summed to obtain a total PAED scale score, children whose PAED score ≥ 10 was considered to have emergence delirium [3].
Mechanical Ventilation
Galileo ventilators (Hamilton Medical, Rhäzuns, Switzerland) with adaptive pressure control ventilation-synchronized intermittent mandatory ventilation (APV-SIMV) were used for all the patients. Continuous positive airway pressure (CPAP) mode was used to wean the patients from mechanical ventilation, and extubation was considered when the control breath rate was zero and support pressure was less than 8 cm H2O.
Statistical Analysis
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) software version 16.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were presented as the absolute and percentage values, and continuous variables were presented as the mean ± standard deviation. Continuous variables with an asymmetrical distribution (skewed distributions) were presented as the median values (interquartile ranges). Disease constituent ratio and delirium were compared using Fisher’s exact test for binary categorical variables. Comparisons between the averages of continuous variables were assessed using Student’s t tests for unpaired data or the Mann–Whitney two-sample statistic, as appropriate. A P value of <0.05 was considered statistically significant.
Results
Demographics
A total of 187 consecutive patients were included in our analysis. There were 82 patients in group Dex and 105 patients in group Mid. In the Dex group, five cases were excluded because two cases had data missing and three cases had sinus bradycardia during treatment. The three cases of sinus bradycardia occurred between 30 min to 1 h after the anesthesia beginning, with a heart rate of 90–100 bpm, and this was considered to be related to the sedative drugs; therefore, we replaced Dex/fentanyl with midazolam 0.5–1 μg kg−1 min−1 for about 30 min, and the heart rates restored to 130–150 times/min. In the Mid group, eight cases were excluded because four cases had data missing and four cases had liver damage (child - Pugh class to C), and in order to avoid drug side effects, we replaced Mid/fentanyl with diazepam 1 mg kg−1 and phenobarbital 5 mg kg−1.
The results of the demographic data analysis revealed no significant differences in the baseline demographics (sex, age, and weight) and clinical characteristics (surgical indication, cardiopulmonary bypass time, systolic pulmonary artery pressure, RACHS-1 categories, and PRISM III-24 scores) between the two groups (Table 1).
Sedation/Analgesia Regimen
In the operating room, all the patients received a balanced anesthetic using a combination of sufentanil, midazolam, propofol, and inhalational isoflurane (at 0.5–1 minimum alveolar concentration [MAC]). Overall, sufentanil and midazolam were the most frequently administered bolus doses in both the groups. There was no difference in the total dose of sufentanil, midazolam, and propofol infusions between group Dex and group Mid (Table 2).
Clinical Outcomes
Ventilation outcomes: The duration of the sedation (median [IQR], 30 (22–46) h vs. 38 (23–51) h, P = 0.079) was similar in both the groups. Group Dex patients spent less time on a mechanical ventilator than group Mid patients [median (IQR), 30 (24–46) h vs 38(25–55) h, P = 0.067], although this difference was not significant. Reintubation was required in two patients (2.6 %) in group Dex and two patients (2.1 %) in group Mid (P = 0.815; Table 3).
In the CICU, group Dex patients required significantly lower doses of adjunctive sedative/analgesic drugs than group Mid patients [median (IQR): midazolam, 0.30 (0.18–0.43) mg kg−1 vs. 0.50 (0.30–0.60) mg kg−1, P = 0.007; morphine, 0.10 (0.05–0.20) vs. 0.20 (0.10–0.40), P < 0.001, Table 3].
The PAED scale (5.2 ± 5.3 in group Dex vs. 7.1 ± 5.2 in group Mid, P = 0.016; Table 3) and the incidence of delirium (18.2 % in group Dex vs. 32.0 % in group Mid, P = 0.039; Table 3) was significantly lower among the group Dex patients than among group Mid patients. Furthermore, there was no difference in the length of the CICU stay (median [interquartile, IQR], 4 (3–5) d in group Dex vs. 4 (3–5) d in group Mid, P = 0.686), hospital stay (median [IQR], 8 (7–9) d in group Dex vs. 8 (7–10) d in group Mid, P = 0.914), and mortality rate (2.6 % in group Dex vs. 3.1 % in group Mid, P = 0.846).When the patients were subgrouped according to their ages, a statistical difference in “Incidence of emergence delirium” was demonstrated in the subgroup of children ≤2 years old (P = 0.040); the PAED scale was also different in both groups but not significantly (Table 4).
Discussion
This study compared two postoperative sedation regimens in children with PAH: dexmedetomidine/fentanyl and midazolam/fentanyl sedation. Lack of sedation increases coughing, hypoxemia, and hypercarbia causing dangerous rises in pulmonary artery pressures; therefore, deep sedation was implemented in our department for these patients, and this was the reason we selected children with PAH for inclusion in the study.
Over 50 % of the children in this study received the sedation treatment for more than 24 h. There were no significant differences in the durations of sedation and ventilator use between the two groups. However, the dexmedetomidine/fentanyl treatment group required significantly lower doses of additional sedative/analgesic treatment and had lower incidence of delirium than the midazolam/fentanyl group, suggesting that the former is a more effective sedative treatment approach. Maybe this is consistent with the mechanism of action, as Dex combines sedative, analgesic, and anxiolytic effects and may induce a sedative state similar to physiological sleep without respiratory depression [10].
Prolonged administration of benzodiazepines and/or opioids to children may induce physiological dependence and withdrawal symptoms [10]. Few studies have evaluated withdrawal symptoms from prolonged infusions of Dex [24]. A recent study [4] has suggested that tachycardia, transient hypertension, and agitation are frequently observed in pediatric cardiac patients after discontinuing prolonged infusions of Dex. The SEDCOM study [18] showed that Dex significantly reduced the incidence of delirium when compared with midazolam. Ibacache et al. [18] reported that a single injection of Dex could reduce the incidence of delirium in children following sevoflurane anesthesia. These results suggest that Dex treatment reduced the incidence of adverse reactions while demonstrating adequate sedative effects. Our study showed that the incidence of delirium was significantly lower in the Dex treatment group, which is consistent with previous studies. When the results were compared according to subgroups divided by age group, the <2 years group showed a larger difference in incidence of delirium between the Dex and Mid groups, and this difference was significant while the subjects over 2 years did not show a significant difference, so the results are age related.
This study had some limitations. First, a more robust study would be a randomized controlled trial rather than the retrospective study performed here. This study relied on random allocation to groups by different physicians who chose either Dex or midazolam for treatment of patients with similar clinical characteristics; to our knowledge there was no medical reason to prefer one method over the other so this was relatively random, but it will have introduced some bias into the study. Second, although the composite PAED score had been shown to be a reliable and valid measure of ED in children [20], we noticed that despite having five objective criteria, the individual score for each item was still rather subjective. For example, it may be difficult to decide whether a child makes ‘extremely’ or ‘very much’ eye contact with the caregiver or ‘quite a bit’ of eye contact, and we tried to avoid major differences by fully explaining all criteria and their measurements to the evaluating nurses, but this also may have introduced some bias into the study. The PAED score has been validated in children over 2 years, but is not often used in those below 1 year, so it must be noted that validation of PAED score in these patients needs to be undertaken. The PAED score was also developed for use in the recovery room; in our hospital, it is standard procedure for all children undergoing cardiac surgery to be sent to ICU for recovery rather than stay in the recovery room. The use of PAED in ICU has also been validated by other studies [2, 11]. We also did not compare mPAP that classified these patients as PAH, which was estimated by echocardiography, with the mPAP data during the operation or postoperation because these were measured directly by arterial sensor in the operating theater and therefore could not be accurately compared. It is impossible for all these children to complete cardiac catheterization before surgery, so the most practical results for diagnosis are echocardiography in China.
In summary, this study found no differences in the durations of the sedation treatment, mechanical ventilator use, and CICU stay between the children with PAH who were administered dexmedetomidine/fentanyl and midazolam/fentanyl; however, dexmedetomidine/fentanyl treatment reduced the required dosage of additional sedative/analgesic drugs and the incidence of delirium. Thus, Dex may be useful for the postoperative treatment of children with PAH.
References
Awori MN, Ogendo SW (2008) Rachs-1 system in risk stratification for congenital heart disease surgery outcome. East Afr Med J 85(1):36–38
Blankespoor RJ, Janssen NJ, Wolters AM, Van Os J, Schieveld JN (2012) Post-hoc revision of the pediatric anesthesia emergence delirium rating scale: clinical improvement of a bedside-tool? Minerva Anestesiol 78(8):896–900
Bong CL, Ng AS (2009) Evaluation of emergence delirium in Asian children using the Pediatric Anesthesia Emergence Delirium Scale. Paediatr Anaesth 19(6):593–600. doi:10.1111/j.1460-9592.2009.03024.x
Burbano NH, Otero AV, Berry DE, Orr RA, Munoz RA (2012) Discontinuation of prolonged infusions of dexmedetomidine in critically ill children with heart disease. Intensiv Care Med 38(2):300–307. doi:10.1007/s00134-011-2441-8
Fagin A, Palmieri T, Greenhalgh D, Sen S (2012) A comparison of dexmedetomidine and midazolam for sedation in severe pediatric burn injury. J Burn Care Res 33(6):759–763. doi:10.1097/BCR.0b013e318254d48e
Fischer LG, Van Aken H, Burkle H (2003) Management of pulmonary hypertension: physiological and pharmacological considerations for anesthesiologists. Anesth Analg 96(6):1603–1616
Freire AX, Afessa B, Cawley P, Phelps S, Bridges L (2002) Characteristics associated with analgesia ordering in the intensive care unit and relationships with outcome. Crit Care Med 30(11):2468–2472. doi:10.1097/01.CCM.0000034695.18650.2A
Friesen RH, Williams GD (2008) Anesthetic management of children with pulmonary arterial hypertension. Paediatr Anaesth 18(3):208–216. doi:10.1111/j.1460-9592.2008.02419.x
Gupta P, Whiteside W, Sabati A, Tesoro TM, Gossett JM, Tobias JD et al (2012) Safety and efficacy of prolonged dexmedetomidine use in critically ill children with heart disease*. Pediatr Crit Care Med 13(6):660–666. doi:10.1097/PCC.0b013e318253c7f1
Hosokawa K, Shime N, Kato Y, Taniguchi A, Maeda Y, Miyazaki T et al (2010) Dexmedetomidine sedation in children after cardiac surgery. Pediatr Crit Care Med 11(1):39–43. doi:10.1097/PCC.0b013e3181b062d7
Janssen NJ, Tan EY, Staal M, Janssen EP, Leroy PL, Lousberg R et al (2011) On the utility of diagnostic instruments for pediatric delirium in critical illness: an evaluation of the Pediatric Anesthesia Emergence Delirium Scale, the Delirium Rating Scale 88, and the Delirium Rating Scale-Revised R-98. Intensive Care Med 37(8):1331–1337. doi:10.1007/s00134-011-2244-y
Jenkins I (2002) The provision of analgesia and sedation in the PICU: current practice and recent advances. Pediatr Anesth 12(9):837–839
Koruk S, Mizrak A, Kaya Ugur B, Ilhan O, Baspinar O, Oner U (2010) Propofol/dexmedetomidine and propofol/ketamine combinations for anesthesia in pediatric patients undergoing transcatheter atrial septal defect closure: a prospective randomized study. Clin Ther 32(4):701–709. doi:10.1016/j.clinthera.2010.04.010
Le KN, Moffett BS, Ocampo EC, Zaki J, Mossad EB (2011) Impact of dexmedetomidine on early extubation in pediatric cardiac surgical patients. Intensive Care Med 37(4):686–690. doi:10.1007/s00134-011-2140-5
Playfor S, Jenkins I, Boyles C, Choonara I, Davies G, Haywood T et al (2006) Consensus guidelines on sedation and analgesia in critically ill children. Intensive Care Med 32(8):1125–1136. doi:10.1007/s00134-006-0190-x
Pollack MM, Patel KM, Ruttimann UE (1996) PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 24(5):743–752
Riker RR, Fraser GL (2005) Adverse events associated with sedatives, analgesics, and other drugs that provide patient comfort in the intensive care unit. Pharmacotherapy 25(5 Pt 2):8S–18S
Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F et al (2009) Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 301(5):489–499. doi:10.1001/jama.2009.56
Siddappa R, Riggins J, Kariyanna S, Calkins P, Rotta AT (2011) High-dose dexmedetomidine sedation for pediatric MRI. Paediatr Anaesth 21(2):153–158. doi:10.1111/j.1460-9592.2010.03502.x
Sikich N, Lerman J (2004) Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology 100(5):1138–1145
Smania MC, Piva JP, Garcia PC (2008) Dexmedetomidine in anesthesia of children submitted to videolaparoscopic appendectomy: a double-blind, randomized and placebo-controlled study. Rev Assoc Med Bras 54(4):308–313
Tobias JD, Berkenbosch JW (2004) Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam. South Med J 97(5):451–455
Twite MD, Rashid A, Zuk J, Friesen RH (2004) Sedation, analgesia, and neuromuscular blockade in the pediatric intensive care unit: survey of fellowship training programs. Pediatr Crit Care Med 5(6):521–532. doi:10.1097/01.PCC.0000144710.13710.2E
Weber MD, Thammasitboon S, Rosen DA (2008) Acute discontinuation syndrome from dexmedetomidine after protracted use in a pediatric patient. Paediatr Anaesth 18(1):87–88. doi:10.1111/j.1460-9592.2007.02377.x
Conflict of interest
The authors declare that they have no competing interests.
Ethical standard
This study was approved by the Ethics Committee of Chengdu Military General Hospital (201109).
Human rights statement
For this type of study (retrospective studies), formal consent is not required.
Author information
Authors and Affiliations
Corresponding author
Additional information
Li Jiang, Sheng Ding, Hongtao Yan have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jiang, L., Ding, S., Yan, H. et al. A Retrospective Comparison of Dexmedetomidine Versus Midazolam for Pediatric Patients with Congenital Heart Disease Requiring Postoperative Sedation. Pediatr Cardiol 36, 993–999 (2015). https://doi.org/10.1007/s00246-015-1110-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-015-1110-z