Abstract
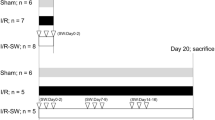
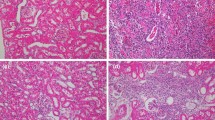
Although the efficacy of extracorporeal shock wave lithotripsy (ESWL) has been well established within the literature, debate continues on the safety of the procedure while focusing on cellular injury and its long-term consequences. Here, we describe the role of neutrophil elastase (NE) in ESWL-related rat kidney damage and investigate the protective effects of sivelestat, an inhibitor of NE, during the early and late phases. Four groups including control, ESWL alone, ESWL with sivelestat 50 mg/kg and ESWL with treatment of 100 mg/kg, each consisting of ten rats were created. Biochemical parameters of kidney function and damage and immunohistopathological findings were compared in the early (72 h after ESWL) and late (1 week after ESWL) periods between the groups. During the early period, serum and urine creatinine levels and urine kidney injury molecule-1 (KIM-1) levels and the KIM-1/creatinine ratio increased in rats treated with ESWL compared to the control group. Furthermore, increased tissue inflammation, ductal dilatation and hemorrhage, and glomerular, tubular, and interstitial damage with increased NE staining were also detected in the ESWL treatment group. During the late phase, although urine KIM-1 levels remained stable at high levels, other parameters showed significant improvements. On the other hand, the administration of sivelestat 50 mg/kg decreased serum creatinine and urine KIM-1 and KIM-1/creatinine levels significantly in rats treated with ESWL, during the early and late periods. Significant decreases in tissue inflammation, tubular, and interstitial tissue damage were also observed during the early period. In conclusion, ESWL-related kidney tissue damage occurs primarily during the early period, and NE is involved in this process. On the other hand, the NE inhibitor sivelestat attenuated this ESWL-induced kidney damage.

Similar content being viewed by others
Availability of data and material
The data are not publicly available.
Code availability
Not applicable.
References
Chaussy C, Brendel W, Schmiedt E (1980) Extracorporeally induced destruction of kidney stones by shock waves. Lancet 2:1265–1268. https://doi.org/10.1016/s0140-6736(80)92335-1
Chaussy C, Schmiedt E, Jocham D, Brendel W, Forssmann W, Walther V (1982) First clinical experience with extracorporeally induced destruction of kidney stones by shock waves. J Urol 127:417–420. https://doi.org/10.1016/s0022-5347(17)53841-0
Reynolds LF, Kroczak T, Pace KT (2018) Indications and contraindications for shock wave lithotripsy and how to improve outcomes. Asian J Urol 5:256–263. https://doi.org/10.1016/j.ajur.2018.08.006
Türk C, Neisius A, Petrik A et al (2021) EAU guidelines on urolithiasis, from EAU guidelines office. https://uroweb.org/guideline/urolithiasis/. Accessed 10 Aug 2021
Quhal F, Seitz C (2021) Guidelines: urolithiasis. Curr Opin Urol 31:125–129. https://doi.org/10.1097/MOU.0000000000000855
El-Nahas AR, Elsaadany MM, Taha DE, Elshal AM, El-Ghar MA, Ismail AM et al (2017) A randomised controlled trial evaluating renal protective effects of selenium with vitamins A, C, E, verapamil, and losartan against lithotripsy-induced extracorporeal shockwave renal injury. BJU Int 119:142–147. https://doi.org/10.1111/bju.13667
Chung JM, Park BK, Kim JH, Lee HJ, Lee SD (2018) Impact of repeated extracorporeal shock wave lithotripsy on the prepubertal rat kidney. Urolithiasis 46:549–558. https://doi.org/10.1007/s00240-017-1011-0
Recker F, Rübben H, Bex A, Constantinides C (1989) Morphological changes after ESWL in the rat kidney. Urol Res 17:229–233. https://doi.org/10.1007/BF00262598
Kira VM, Fagundes DJ, Bandeira CO, Kaufman O, Fagundes AT, Ortiz V (2008) Effects of repeated extracorporeal shock waves on kidney apoptosis of normal and diabetic rats. Int Braz J Urol 34:91–96. https://doi.org/10.1590/s1677-55382008000100013
Lee HY, Yang YH, Shen JT, Jang MY, Shih PM, Wu WJ et al (2013) Survey of risk factors for renal hematoma induced by extracorporeal shock wave lithotripsy. J Endourol 27:763–767. https://doi.org/10.1089/end.2012.0619
McAteer JA, Evan AP (2008) The acute and long-term adverse effects of shock wave lithotripsy. Semin Nephrol 28:200–213. https://doi.org/10.1016/j.semnephrol.2008.01.003
Freund JB, Colonius T, Evan AP (2007) A cumulative shear mechanism for tissue damage initiation in shock-wave lithotripsy. Ultrasound Med Biol 33:1495–1503. https://doi.org/10.1016/j.ultrasmedbio.2007.03.001
Evan AP, McAteer JA, Connors BA, Blomgren PM, Lingeman JE (2007) Renal injury during shock wave lithotripsy is significantly reduced by slowing the rate of shock wave delivery. BJU Int 100:624–627. https://doi.org/10.1111/j.1464-410X.2007.07007.x (discussion 627–628)
Clark DL, Connors BA, Evan AP, Willis LR, Handa RK, Gao S (2009) Localization of renal oxidative stress and inflammatory response after lithotripsy. BJU Int 103:1562–1568. https://doi.org/10.1111/j.1464-410X.2008.08260.x
Dziegala M, Krajewski W, Kolodziej A, Dembowski J, Zdrojowy R (2018) Evaluation and physiopathology of minor transient shock wave lithotripsy—induced renal injury based on urinary biomarker levels. Cent Eur J Urol 71:214–220. https://doi.org/10.5173/ceju.2018.1629
Sarica K, Kosar A, Yaman O, Beduk Y, Durak I, Gogus O, Kavukcu M (1996) Evaluation of ischemia after ESWL: detection of free oxygen radical scavenger enzymes in the renal parenchyma subjected to high-energy shock waves. Urol Int 57:221–223. https://doi.org/10.1159/000282918
Goktas C, Coskun A, Bicik Z, Horuz R, Unsal I, Serteser M (2012) Evaluating ESWL-induced renal injury based on urinary levels of TNF-alpha, IL-1alpha, and IL-6. Urol Res 40:569–573. https://doi.org/10.1007/s00240-012-0467-1
Bronze-da-Rocha E, Santos-Silva A (2018) Neutrophil elastase inhibitors and chronic kidney disease. Int J Biol Sci 14:1343–1360. https://doi.org/10.7150/ijbs.26111
Alam SR, Newby DE, Henriksen PA (2012) Role of the endogenous elastase inhibitor, elafin, in cardiovascular injury: from the epithelium to the endothelium. Biochem Pharmacol 83:695–704. https://doi.org/10.1016/j.bcp.2011.11.003
Fujie K, Shinguh Y, Inamura N, Yasumitsu R, Okamoto M, Okuhara M (1999) Release of neutrophil elastase and its role in tissue injury in acute inflammation: effect of the elastase inhibitor, FR134043. Eur J Pharmacol 374:117–125. https://doi.org/10.1016/s0014-2999(99)00268-x
Nakazawa D, Marschner JA, Platen L, Anders HJ (2018) Extracellular traps in kidney disease. Kidney Int 94:1087–1098. https://doi.org/10.1016/j.kint.2018.08.035
Ishii T, Doi K, Okamoto K, Imamura M, Dohi M, Yamamoto K et al (2010) Neutrophil elastase contributes to acute lung injury induced by bilateral nephrectomy. Am J Pathol 177:1665–1673. https://doi.org/10.2353/ajpath.2010.090793
Polverino E, Rosales-Mayor E, Dale GE, Dembowsky K, Torres A (2017) The role of neutrophil elastase inhibitors in lung diseases. Chest 152:249–262. https://doi.org/10.1016/j.chest.2017.03.056
Pu S, Wang D, Liu D, Zhao Y, Qi D, He J, Zhou G (2017) Effect of sivelestat sodium in patients with acute lung injury or acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. BMC Pulm Med 17:148. https://doi.org/10.1186/s12890-017-0498-z
Li G, Jia J, Ji K, Gong X, Wang R, Zhang X et al (2016) The neutrophil elastase inhibitor, sivelestat, attenuates sepsis-related kidney injury in rats. Int J Mol Med 38:767–775. https://doi.org/10.3892/ijmm.2016.2665
Voisin MB, Leoni G, Woodfin A, Loumagne L, Patel NS, Di Paola R et al (2019) Neutrophil elastase plays a non-redundant role in remodeling the venular basement membrane and neutrophil diapedesis after ischemia/reperfusion injury. J Pathol 248:88–102. https://doi.org/10.1002/path.5234
Matsuzaki K, Hiramatsu Y, Homma S, Sato S, Shigeta O, Sakakibara Y (2005) Sivelestat reduces inflammatory mediators and preserves neutrophil deformability during simulated extracorporeal circulation. Ann Thorac Surg 80:611–617. https://doi.org/10.1016/j.athoracsur.2005.02.038
von Nussbaum F, Li VM (2015) Neutrophil elastase inhibitors for the treatment of (cardio)pulmonary diseases: in clinical testing with pre-adaptive pharmacophores. Bioorg Med Chem Lett 25:4370–4381. https://doi.org/10.1016/j.bmcl.2015.08.049
Takemasa A, Ishii Y, Fukuda T (2012) A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. Eur Respir J 40:1475–1482. https://doi.org/10.1183/09031936.00127011
Kohira S, Oka N, Inoue N, Itatani K, Kitamura T, Horai T et al (2014) Effect of additional preoperative administration of the neutrophil elastase inhibitor sivelestat on perioperative inflammatory response after pediatric heart surgery with cardiopulmonary bypass. Artif Organs 38:1018–1023. https://doi.org/10.1111/aor.12311
Nomura N, Asano M, Saito T, Nakayama T, Mishima A (2013) Sivelestat attenuates lung injury in surgery for congenital heart disease with pulmonary hypertension. Ann Thorac Surg 96:2184–2191. https://doi.org/10.1016/j.athoracsur.2013.07.017
Tagami T, Tosa R, Omura M, Fukushima H, Kaneko T, Endo T et al (2014) Effect of a selective neutrophil elastase inhibitor on mortality and ventilator-free days in patients with increased extravascular lung water: a post hoc analysis of the PiCCO Pulmonary Edema Study. J Intensive Care 2:67. https://doi.org/10.1186/s40560-014-0067-y
Iwata K, Doii A, Ohji G, Oka H, Oba Y, Takimoto K et al (2010) Effect of neutrophil elastase inhibitor (sivelestat sodium) in the treatment of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): a systematic review and meta-analysis. Intern Med 49:2423–2432. https://doi.org/10.2169/internalmedicine.49.4010
Aikawa N, Kawasaki Y (2014) Clinical utility of the neutrophil elastase inhibitor sivelestat for the treatment of acute respiratory distress syndrome. Ther Clin Risk Manag 5:621–629. https://doi.org/10.2147/TCRM.S65066
Acknowledgements
We would like to thank the director of our animal laboratory, Duygu Sultan Oran, and her team for their valuable support.
Funding
None.
Author information
Authors and Affiliations
Contributions
AC, EK, and AS designed the study. AC, SZ, SG, EK, and AS coordinated the study. AC and MZT designed and ran the literature search. AC, SZ, SG, SA, SO, and SHK collected and extracted data. AC, MZT, SA, SO and SHK performed the data analysis and data interpretation. AC, MZT, and EY drafted the manuscript. SHK and SO contributed to the writing. EY, EK, and AS supervised the work and critically reviewed the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they had no conflict of interest or competing interests.
Ethical approval
All animal experiments and procedures were in accordance with the principles of laboratory animal care and were approved by the Institutional Animal Care and Use Committee (approval ID: 2017/40).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Colakerol, A., Suzan, S., Temiz, M.Z. et al. Tissue neutrophil elastase contributes to extracorporeal shock wave lithotripsy-induced kidney damage and the neutrophil elastase inhibitor, sivelestat, attenuates kidney damage with gratifying immunohistopathological and biochemical findings: an experimental study. Urolithiasis 50, 103–112 (2022). https://doi.org/10.1007/s00240-021-01287-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-021-01287-x