Abstract
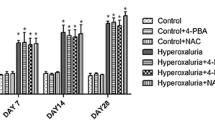
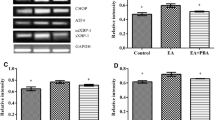
Hyperoxaluria is characterized by an increased excretion of urinary oxalate which is caused by inherited disorders or high oxalate intake leading to renal stone ailment. Until date, reactive oxygen species and inflammation has been convicted for the progression of kidney stones for which antioxidant therapy has been employed. However, recent studies have linked the association of endoplasmic reticulum stress and oxidative imbalance in the progression of renal diseases. Considering oxidative stress being at forefront in causing hyperoxaluric consequences, current study was designed to correlate the impact of hyperoxaluria and regulation of oxidative imbalance via inhibition of endoplasmic reticulum stress by 4-phenylbutyric acid (4-PBA). Male wistar rats were subdivided into three groups, i.e., normal control (C), hyperoxaluric rats given ethylene glycol (EG), and hyperoxaluric rats treated with 4-PBA (EG + PBA). After 28 days of treatment, assessment of antioxidant defence system, inflammation, ER stress, and subsequent unfolded protein response was studied in renal tissue. It was found that the hyperoxaluric insult led to a marked damage to the renal tissue resulting in compromised antioxidant levels, upregulation of ER stress markers along with a steep surge in the extent of inflammation. However, 4-PBA treatment significantly curtailed the deleterious effects of hyperoxaluria by lowering down the level of stress markers as well as normalizing the antioxidant defence enzymes. Therefore, chemical chaperones can be deemed as a new class of drugs for the treatment of hyperoxaluric induced renal damage.





Similar content being viewed by others
References
Lorenz EC, Michet CJ, Milliner DS, Lieske JC (2013) Update on oxalate crystal disease. Curr Rheumatol Rep 15:1–9
Moe OW (2006) Kidney stones: pathophysiology and medical management. Lancet 367:333–344
Joshi S, Wang W, Peck AB, Khan SR (2015) Activation of the NLRP3 inflammasome in association with calcium oxalate crystal induced reactive oxygen species in kidneys. J Urol 193:1684–1691
Khan SR (2013) Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol 189:803–811
Khan SR (2005) Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res 33:349–357
Selvam R (2002) Calcium oxalate stone disease: role of lipid peroxidation and antioxidants. Urol Res 30:35–47
Kaneko K, Kobayashi R, Yasuda M, Izumi Y, Yamanobe T, Shimizu T (2012) Comparison of matrix proteins in different types of urinary stone by proteomic analysis using liquid chromatography–tandem mass spectrometry. Int J Urol 19:765–772
Knorle R, Schnierle P, Koch A, Buchholz NP, Hering F, Seiler H, Ackermann T, Rutishauser G (1994) Tamm–Horsfall glycoprotein: role in inhibition and promotion of renal calcium oxalate stone formation studied with Fourier-transform infrared spectroscopy. Clin Chem 40:1739–1743
Jaggi M, Nakagawa Y, Zipperle L, Hess B (2007) Tamm–Horsfall protein in recurrent calcium kidney stone formers with positive family history: abnormalities in urinary excretion, molecular structure and function. Urol Res 35:55–62
Bhardwaj R, Bhardwaj A, Tandon C, Dhawan DK, Bijarnia RK, Kaur T (2017) Implication of hyperoxaluria on osteopontin and ER stress mediated apoptosis in renal tissue of rats. Exp Mol Pathol 102:384–390
Marciniak SJ, Ron D (2006) Endoplasmic reticulum stress signaling in disease. Physiol Rev 86:1133–1149
Chambers JE, Marciniak SJ (2014) Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 2. Protein misfolding and ER stress. Am J Physiol Cell Physiol 307:C657–C670
Engin F, Hotamisligil GS (2010) Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes Metab 12:108–115
Ricobaraza A, Cuadrado-Tejedor M, Pérez-Mediavilla A, Frechilla D, Del Río J, García-Osta A (2009) Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer’s disease mouse model. Neuropsychopharmacology 34:1721–1732
Luo T, Chen B, Wang X (2015) 4-PBA prevents pressure overload-induced myocardial hypertrophy and interstitial fibrosis by attenuating endoplasmic reticulum stress. Chem Biol Interact 242:99–106
Cao AL, Wang L, Chen X, Wang YM, Guo HJ, Chu S, Liu C, Zhang XM, Peng W (2016) Ursodeoxycholic acid and 4-phenylbutyrate prevent endoplasmic reticulum stress-induced podocyte apoptosis in diabetic nephropathy. Lab Investig 96:610–622
Montane J, de Pablo S, Castaño C, Rodríguez-Comas J, Cadavez L, Obach M, Visa M, Alcarraz-Vizán G, Sanchez-Martinez M, Nonell-Canals A, Parrizas M (2017) Amyloid-induced β-cell dysfunction and islet inflammation are ameliorated by 4-phenylbutyrate (PBA) treatment. FASEB J 31:5296–5306
Brusilow SW, Maestre NE (1996) Urea cycle disorders: diagonosis, pathophysiology, and therapy. Adv Pediatr 43:127–170
Bhardwaj R, Tandon C, Dhawan DK, Kaur T (2017) Effect of endoplasmic reticulum stress inhibition on hyperoxaluria induced oxidative stress: influence on cellular ROS sources. World J Urol 35:1955–1965
Ashok P, Koti BC, Vishwanathswamy AHM (2010) Antiurolithiatic and antioxidant activity of Mimusops elengi on ethylene glycol-induced urolithiasis in rats. Indian J Pharmacol 42:380–383
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582:67–78
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Wills ED (1966) Mechanisms of lipid peroxide formation in animal tissues. Biochem J 99:667–676
Kono Y (1978) Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195
Luck H (1963) Catalase. In: Bergmeyer HW (ed) Methods of enzymatic analysis, vol 3, Academic Press, New York, pp 885–888
Khan SR (2014) Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol 3:256–276
Mulay SR, Evan A, Anders HJ (2014) Molecular mechanisms of crystal-related kidney inflammation and injury. Implications for cholesterol embolism, crystalline nephropathies and kidney stone disease. Nephrol Dial Transplant 29:507–514
Chaudhari N, Talwar P, Parimisetty A, d’Hellencourt CL, Ravanan P (2014) A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci 8:1–15
Kahles F, Hannes M, Bruemmer FD (2014) Osteopontin: a novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab 3:384–393
Vaidya VS, Bonventre JV (2006) Mechanistic biomarkers for cytotoxic acute kidney injury. Expert Opin Drug Metab Toxicol 2:697–713
Khandrika L, Koul S, Meacham RB, Koul HK (2012) Kidney injury molecule-1 is up-regulated in renal epithelial cells in response to oxalate in vitro and in renal tissues in response to hyperoxaluria in vivo. PLoS One 7:e44174
Lushchak VI (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 224:164–175
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O (2012) Oxidative stress and antioxidant defense. World Allergy Organ J 5:9–19
Funding
This study was funded by UGC-SAP (F.4-1/2015/DSA-1 (Sap-4)) and DST-FIST (SR/FST/LS1-645).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards as approved by the institutional Animal house Ethics Committee and were in accordance with the guidelines for care of laboratory Animals (PU/IAEC/S/16/66). This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was not applicable, as the current study does not include human participants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
240_2018_1064_MOESM1_ESM.docx
Supplementary Table 1. A) The body weight of rats was recorded before the start of respective dose and after completion of period of 28 days; the rats were weighed to observe the change in the body weight. B) Change in the weight of rats at day 1 (Initial weight) and day 28 (Final Weight). Values are expresses as mean ± S.D. (n=5) (*p<0.05 represents significant difference with respect to EG group). (DOCX 14 KB)
Rights and permissions
About this article
Cite this article
Randhawa, R., Bhardwaj, R. & Kaur, T. Amelioration of hyperoxaluria-induced kidney dysfunction by chemical chaperone 4-phenylbutyric acid. Urolithiasis 47, 171–179 (2019). https://doi.org/10.1007/s00240-018-1064-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-018-1064-8