Abstract
Background
Preclinical training in microsurgery usually proceeds through steps of increasing difficulty. Traditionally, advanced training is carried out on living animal models which best reproduce the clinical scenario, but recently, the increasing interest in animal rights has led to a greater development and spread of different non-living models for all steps of training.
Methods
The aim of this study was to identify, through a review of the literature, the inanimate models suitable for basic and intermediate/advanced training courses and to evaluate their pros and cons. The search was carried out exclusively through the PubMed database, with "microsurgery" or “supermicrosurgery” and ("training" or "non-living model") as keywords in the "title and/or abstract" fields. The filters used were: publication date (2010–2022) and species (other animals). The study was done following the PRISMA 2020 checklist criteria.
Results
A total number of 398 articles were initially screened. Following abstract review, 75 articles were selected, and 51 articles were chosen following full text review. Several non-living models are available for training on fine dissection and microsurgical technique. Among the non-animal models, food and synthetic materials (silicone tubes and latex gloves) were predominantly used. Among the non-living animals, the chicken was the most frequently used animal followed by the pig and the rat. Non-living animal mainly focus on vascular sutures on vessels of different vessels, including very small vessels for supermicrosurgery.
Conclusions
The results of this study have shown that many different non-living models are available not only for basic microsurgical training, but also for intermediate training. These models allow to improve microsurgical and supermicrosurgical skills, simultaneously reducing the use of living animals, according to the “3 R” principle. Their main limitation is that due to their characteristics, as tissue consistency and the absence of a pumping flow, they do not provide a realistic experience as that on living animals, which are still the reference for the final phases of microsurgical training.
Level of evidence:Not ratable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preclinical training is essential during microsurgical education. While the traditional method for learning surgery is based on the “experience principle”, gaining skills assisting and operating on patients under supervision of experienced surgeons, microsurgical procedures require a high level of technical skills, manual dexterity and mastering at least the basic microsurgical techniques before moving on clinical practice. Microsurgical education is still challenging, due to the long learning curve and the difficulties to reproduce in a preclinical setting all the pathophysiological phenomena that the trainee should learn to manage. Microsurgical training usually proceeds through steps of increasing difficulty and practical courses of different levels are available: basic courses on food or synthetic materials, intermediate and advanced courses on non-living or living animal [1].
The increasing interest in animal rights [2] has led to greater development of non-living models for all steps of training [3]. Despite the training on rats remains the gold standard for microsurgery, non-living models represent a valid alternative for at least the basic training and their potential is still underexploited. They allow to learn: how to handle microsurgical instruments, to tie and square the microsurgical knots, to improve manual dexterity and tissue dissection, to practice with nerve and vascular sutures. They make the training easily accessible. They don’t require any Ethical Committee approbation, laboratories for animals, veterinarians or anaesthesia, nor animal sacrifice. They are cost effective, with materials simply to find and exercises easy to replicate [4]. However, the absence of a continuous blood flow and intravascular trombi formation, limit their application for intermediate/advanced steps of training. Otherwise, living animal better simulate the microsurgical clinical practice allowing to perform tissues dissection, the raise of tissue flaps and vascular anastomosis on tissues similar to the human’s ones. They imply ethical issues about training on living animal and require following multiple ethical norms. They need dedicated laboratories and personnel, which are not always available and funds to spend for the animals. The training on living models is expensive and more complicated to plan and organize. Indeed, non-living models (animal and synthetic ones) are useful not to completely replace living models, but to reduce as much as possible the number of living animals required by the more experienced trainees in the final steps of their microsurgical training, in line with 3R rules (refine, replace and reduction)[2].
Despite living animals are still more popular for microsurgical training, especially for the intermediate/advanced phases, a larger application of non-living ones is desiderable.
The aim of this study was to review recent literature on the inanimate models for microsurgical and supermicrosurgical training and discuss their potential in the different steps of education. By summarizing and discussing the available non-living models, we also aim at providing a reference and further popularize inanimate models for miscrosurgical training.
Materials and methods
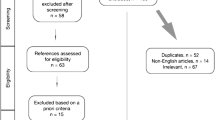
A review of the literature was performed. The search was carried out exclusively through the PubMed database, with "microsurgery" or “supermicrosurgery” and ("training" or "non-living model") as keywords in the "title and/or abstract" fields. The filters used were: publication date (2010–2022) and species (other animals). Search date was 02/04/2022. The works were selected reviewing the abstract or the full text by two reviewers, who indipendently selected the articles and extrapolated the data from each included studies. The inclusion criteria were: articles in English, Italian, Spanish or French concerning non-living models (food, synthetic material or non-living animal model) for microsurgical or supermicrosurgical training. Articles in other languages or concerning only living animal models or fields other than microsurgical/supermicrosurgical training were excluded. Articles selection process flowchart is shown in Fig. 1.
From each article, the following data were recorded: type of synthetic material and/or food, type of non-living animal model, part of the body of the animal, vessels and/or nerves, model of training (microsurgery and/or supermicrosurgery), patency evaluation methods. No ethical approval was required for this study. This manuscript was written following the PRISMA 2020 checklist criteria [5].
Results
Literature search
A total number of 398 articles were initially screened. Following abstract review, 75 articles were selected, and 51 articles were chosen following full text review. The data extrapolated from the 51 papers are shown in Table 1. Most studies focused on microsurgical models, while ten studies [6,7,8,9,10,11,12,13,14,15] focused on supermicrosurgery. Fourteen studies used non-animal models (synthetic materials and food) [7, 8, 16,17,18,19,20,21,22,23,24,25,26,27], twenty-six studies used non-living animal only [9,10,11,12,13,14,15, 28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] and eleven studies used both non-living animal and non-animal models [6, 47,48,49,50,51,52,53,54,55,56].
Non-animal models
Among non-animal models, synthetic materials were predominantly used. The most popular synthetic materials were silicone tubes [6,7,8, 12, 16, 20, 21, 26, 47, 48, 50] and latex gloves [24, 27, 47, 48, 51,52,53,54].
Less frequently employed synthetic materials were needles with an eyelet [19, 22, 51], latex plates [20], vinyl or nitrile gloves [22, 47], latex tubes [25], coloring micro-grids and latex sheets [23], Mepitel™ [51], polyester endovascular prostheses, gelatine, polypropylene fluoropolymer [52] and 3D printed silicone microvessels [56]. Food was used as training material in only three studies [17, 18, 51].
Non-living animals
Among non-living animals, the chicken was the most used animal [6, 9,10,11,12, 14, 15, 31, 32, 35,36,37,38,39, 46,47,48,49,50,51, 53], followed by the pig [13, 28, 29, 40,41,42, 53, 54], the rat [43,44,45, 49, 54], the human [33, 34, 52], and the turkey [30, 47]. In the chickens and the turkeys, the parts mostly used were the thigh and the wing, followed by the leg, the paw, the chest and the neck. In the pig, the head, the trunk and the foot were all used. On rats, exercises mainly focused on vascular suture on the aorta, the femoral vessels, and the neck vessels. The studies reporting training on non-living rats utilized segment of cryopreserved vessels or freshly harvested ones from rat cadavers. In the examined human models, the human placenta [52], the umbilical cord [33] and the lymph node vessels harvested from discarded tissues after neck, axillary or groin dissection were used [34].
Magnification tools and patency assessment
Instead of the microscope, the smartphone or iPad were used for magnification in 3 studies [27, 31, 32].
Patency of the anastomosis was tested in 61% of the studies [9,10,11,12,13, 15, 17, 18, 21,22,23, 26, 28,29,30, 33, 35, 36, 38, 39, 42, 43, 45, 49,50,51,52,53, 55], 57% in non-animal models [17, 18, 21,22,23, 26] and 63% in non-living animals [9,10,11,12,13, 15, 28,29,30, 33, 35, 36, 38, 39, 42, 43, 45, 49,50,51,52,53, 55]. The injection of a coloured solution was the most used method to test the anastomosis [12, 13, 15, 17, 26, 28,29,30, 33, 35, 36, 38, 39, 45, 50, 51, 55] followed by the method of reperfusion with residual endogenous [42, 52] or exogenous [7] blood in non-living animal and the assessment of the patency of the lumen through its direct observation [10, 18, 21] or passing a suture tread inside it [9,10,11].
Discussion
The results show that several non-living models are available for training on microsurgical technique.
Contemporary ethical principles suggest to use models that respect animal rights, according to the “3 R” principles: replacement of the animals with alternative models or with animals with the least neurological development to minimize the pain perceived; reduction of animals used in the experiments; refinement of the animals’ living conditions [2]. Recently, guidelines for a basic microsurgical training course have been elaborated during the IMSS (International Microsurgery Simulation Society) consensus. These guidelines set the minimum requirements for a basic course and the goals to accomplish before starting supervised clinical practice: an ideal microsurgical course should last at least 40 h, with the early stages (first 8 – 24 h) based on non-animal models and each trainee should perform a minimum of 55 anastomoses [3].
The reviewed literature has shown that several exercises aim to develop fine hand–eye coordination, tissue dissection and basic suturing techniques.
Latex gloves and plates are useful to acquire dexterity in suturing and knot tying, allowing to practice in the placement of the stitches on a straight incision [20, 24, 27, 38, 48]. An “evolution” of the latex glove/plate is represented by the surgical gauze: the presence of a thin interweaving fibers adds more complexity to the training, consenting to pass the needle above and below them, improving fine movements and hand–eye coordination [53]. The microsurgery wire’s passages in the eye of sewing needles help to develop dexterity [19]. The dissection of a star-shaped skin off the flesh of the grape may improve fine tissue dissection [51] and catching of flower petals is gainful to improve manual finesse and atraumatic touch [49]. Synthetic tubes and noodles can be helpful to refine suturing skills respectively on vessels and nerves [17, 18, 21].
Particularly, while synthetic tubes permit, due to their hollow body, to practice on microsurgical vessel anastomoses, Japanese noodles are more adequate, for their solid structure, to practice on microsurgical nerve sutures. A recent study has proven that noodles can be considerated a feasible microsurgical training model for surgeons who don’t have access to animal experimentation for its availability, cost-effectiveness and similarity to the rat’s model, with a comparable diameter of about 1.5 mm [17].
Animal cadaveric corpses and organs offer a simulation experience closer to reality if compared to synthetic materials for the more realistic tissue consistency. The most used ones are chicken, rat and pig models [6, 9,10,11,12,13,14,15, 28, 29, 31, 32, 35,36,37,38,39,40,41,42,43,44,45, 47,48,49,50,51, 53,54,55].
While, the most used are animal limbs, peculiar models are the chicken oesophagus and trachea [35], the pig face, [40, 41], splenic and kidney vessels [28, 42], chest wall [29] and the human placenta vessels [33, 52, 53], which represent valid alternatives to make training less monotonous.
The chicken aorta model, for its large diameter (about 4 mm), is useful especially for novice trainees to acquire confidence with vessel anastomoses [39].
Chicken’s lower and upper limb are the most used materials providing vessels ranging from 2 mm to less than 1 mm in diameter, letting to gradually increase the training difficulty. The chicken can be dissected from the thigh using branches of ischiatic vessels [10,11,12], from the leg on collateral branches of the perforating vessel coming off from the medial tibial artery [11] and from the wing on the ulnar artery and its superficial branch, the deep radial artery and the metacarpal artery [6, 14].
The chicken thigh, due to the presence of a clear neurovascular bundle (femoral artery, vein and nerve), allows to practice on both vessel and nerve. Several studies have demonstrated that it provides an objective improvement in microsurgical skills and a reduction of the anastomosis time [36, 50, 57, 58].
The chicken leg permits to raise flaps based on perforators originating from the medial tibial artery, simulating microsurgical perforator flaps in humans [11].
The reimplantation of the chicken paw finger enables to learn the operative steps of digital reimplantation, with vessels suitable for supermicrosurgery[9].
Chicken wing has been used to practice on vessels that have a caliber similar to human ones and to harvest bone flaps [46, 59,60,61,62,63].
For what concerns swine models, thigh offers vessels with a diameter of about 6 mm. Moreover, the length of vessels is greater than those available in other models, allowing novice microsurgeons to perform multiple anastomoses on larger calibers [64,65,66].
Porcine spleen and coronary vessels have a diameter as low as 0.5 mm and can be used to practice supermicrosurgery[42, 66].
In the porcine foot different size of lymphatic vessels can be used to effectively simulate supermicrosurgerical procedures such as mapping, identification, and dissection of lymphatic vessels, as well as lymphaticovenular anastomosis (LVA) [13].
Furthermore, non-living models make possible a home microsurgical training without the use of the microscope using the magnification of electronic devices such as iPad and smartphone [27, 31, 32], allowing an easy, accessible and cost-effective microsurgical training.
The main advantages of food and synthetic materials are: low cost [20], ease of access [36], speed of execution of the anastomosis [17], possibility to learn to handle the instruments and the microscope [48]. There are some drawbacks: different consistency if compared with biological tissues [42], uselessness to learn dissection techniques [42], less realistic feeling if compared with animal models, absence of adventitia and spasm [17].
The non-living animal described in the literature [33, 34] globally have the advantage to make training easily accessible [38] and to allow: to refine the manual dexterity; to perform a realistic simulation due to the almost identical tactile feeling to microsurgery on human [10]; to maintain the techniques already learned [48], to reduce the number of living animals [6, 47, 53]. They don’t require dedicated laboratories, veterinarians, anaesthesia [10, 36] and do not necessitate preoperative, intraoperative and postoperative care [10]. They also present some disadvantages: the presence of a dense adventitia [36], the absence of a dynamic blood flow [30, 41] and the impossibility to replicate, in a stress-free environment, the elements of decision-making characteristic of the operating room [51]. Overall, they are useful in both basic and more advanced training and some authors argue that they could even replace living models in the setting of advanced microsurgical training [30, 47, 48].
An innovative tool for the initial training of surgeons is Virtual Reality (VR) [67] defined as a combination of a computer with 3D visualization, a head-mounted display and one or more controllers that can provide position tracking and forced or haptic feedback.
Several simulators for microsurgical anastomosis have been developed by different groups/manifacturers but unfortunately, commercial VR simulators are not widely available today [67].
The VR simulation provides many advantages: it can be replicated as many times as needed with possibility of fail-safe attempts, the degree of difficulty can be adjusted depending on trainee’s level of expertise, practice can be performed in a completely stress- and risk-free environment and there’s no need for living models use. In addition, the simulation environment is similar to the real one because both are observed through a narrow optical field [67].
Since more efforts are needed to include VR simulation in standard microsurgical practice, we believe that today non-living models represent the best choice for the basic microsurgical training.
Althought non-living models make the training more accessible, cheap and reduce animal sacrifice, their main drawback is to be less realistic compared to the living animal models.
The absence of a continuous blood flow makes the training untruthfully easier, does not allow the trainee to manage with the pumping flow during the preparation of the operative field, with the loss of blood from the anastomosis after vascular clamps removal nor lets the trainee practice with the common patency test (milking test). Nevertheless, there are several techniques to verify the patency of the anastomosis even in inanimate models, such us: the direct vision of the lumen through cutting the anastomosis [10, 18, 21], the injection of coloured fluid [12, 13, 17, 26, 30, 33, 35, 36, 38, 39, 45, 50, 51] or passing of suture tread inside the lumen [9,10,11]. However, the viability of these models is still limited by the lack of pulsatility and the absence of intraluminal thrombi formation [30]. The impossibility of trombi formation into the vessels of non-living animals makes the evaluation of the patency of the anastomosis less realistic, as in clinical practice trombi formation can occurs at any time after microsurgery, both spontaneously and after a minor trauma. Moreover, microsurgery requires fundamental surgical skills, not only related on performing vascular anastomosis, such as the fine tissue dissection, the control of tissue bleeding, the harvesting of vessels for the anastomosis and the adventectomy, all of them quite difficult or impossible to train on non-living models, especially on the synthetic ones. Despite that, the continuos research in the field of microsurgical training has introduced innovative tools that allow to make inanimate model more real-looking: the use of a peristaltic pump commercially available provides a pulsatile pressure wave and an arterial flow comparable to normal human parameters (60 to 120 mmHg) [68], the application of an extracorporeal perfusion device to rat cadavers with indocyanine green fluorescent infusion make possible a valid assessment of the anastomosis and body perfusion in non-living animal models [69] and also among syntetic models, there is a cheap and effective device made of 3D-printed silicon-containing micro-tubes connected to a pump for fluid flow, simulating the blood circulation [56]. In addition, anatomical studies on non-living animal demostrated that they can be used for practicing with flap raising and tissue dissection [46, 50].
Although the living animals still remain the gold standard in advanced microsurgical training, non-living models represent an element of primary importance in the formation of trainees. Nevertheless, we feel that today the utility of non-living models should be intended differently: their main purpose is not to completely replace living models, but to reduce as much as possible the number of living animals required by the more experienced trainees in the final steps of their training. For this reason, their acceptance as stand-alone training models should be carefully limited to the early and intermediate phases of microsurgical practice, while in the more advanced steps of training they should be used as an additional model to limit animal sacrifice, following the 3R principles. Despite that, the on-going research in the field of simulation, with tools that already try to overcome to non-living models limitations [56, 68, 69], let us hope for a future larger application of non-living models and a possible total replacement of living ones.
This review provides a state of art picture of the advantages and disadvantages of the different non-living models for microsurgical training.
Conclusions
The review of the literature has shown that non-living models, especially non-living animals, are suitable not only for basic microsurgical training, but also for the intermediate phases. The chicken model is a valid alternative to the living animal models [47, 48], as the rat, which today is still considered the gold standard for microsurgical training.
Non-living models are considered both useful to prepare the trainee before passing on living ones [1, 7, 20, 25, 27, 49] and/or such as an alternative to the training on living animals [20, 41, 42, 47, 48, 52], in respect with the 3R principles.
Data availability
The authors confirm that the data supporting the findings of this study ara available within the article and/or its supplementary materials.
References
Tos P, Pedrazzini A (2015) Consiglio direttivo della Società Italiana di Microchirurgia. Introduzione. In: Tos P, Pedrazzini A (eds) Manuale di Microchirurgia, dalle tecniche di base a quelle avanzate. Timeo Editore s.r., Bologna, pp 17–9
Castaldo S (2015) Regola delle 3r per minimizzare l’impatto sull’animale da laboratorio. In: Tos P, Pedrazzini A (eds) Manuale di Microchirurgia, dalle tecniche di base a quelle avanzate. Timeo Editore s.r.l, Bologna, pp 207–211
Ghanem A, Kearns M, Ballestín A, Froschauer S, Akelina Y, Shurey S et al (2020) International microsurgery simulation society (IMSS) consensus statement on the minimum standards for a basic microsurgery course, requirements for a microsurgical anastomosis global rating scale and minimum thresholds for training. Injury 51:S126–S130. https://doi.org/10.1016/j.injury.2020.02.004
Evgeniou E, Walker H, Gujral S (2018) The Role of Simulation in Microsurgical Training. J Surg Educ 75:171–181. https://doi.org/10.1016/j.jsurg.2017.06.032
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Onoda S, Kimata Y, Sugiyama N, Tokuyama E, Matsumoto K, Ota T et al (2016) Analysis of 10-Year Training Results of Medical Students Using the Microvascular Research Center Training Program. J Reconstr Microsurg 32:336–341. https://doi.org/10.1055/s-0035-1568884
Matsumura N, Horie Y, Shibata T, Kubo M, Hayashi N, Endo S (2011) Basic training model for supermicrosurgery: A novel practice card model. J Reconstr Microsurg 27:377–381. https://doi.org/10.1055/s-0031-1281518
Galmiche C, Hidalgo Diaz JJ, Vernet P, Facca S, Menu G, Liverneaux P (2018) Learning of supermicrosurgical vascular anastomosis: MicroChirSim ® procedural simulator versus Anastomosis Training Kit ® procedural simulator. Hand Surg Rehabil 37:20–23. https://doi.org/10.1016/j.hansur.2017.10.236
Athanassopoulos T, Loh CYY (2015) The chicken foot digital replant training model. Hand Surg 20:199–200. https://doi.org/10.1142/S0218810415200026
Chen WF, Eid A, Yamamoto T, Keith J, Nimmons GL, Lawrence WT (2014) A novel supermicrosurgery training model: The chicken thigh. J Plast Reconstr Aesthet Surg 67:973–978. https://doi.org/10.1016/j.bjps.2014.03.024
Cifuentes IJ, Rodriguez JR, Yañez RA, Salisbury MC, Cuadra ÁJ, Varas JE, Dagnino BL (2016) A Novel Ex Vivo Training Model for Acquiring Supermicrosurgical Skills Using a Chicken Leg. J Reconstr Microsurg 32(09):699–705
Zeng W, Shulzhenko NO, Feldman CC, Dingle AM, Poore SO (2018) “blue-Blood”- Infused Chicken Thigh Training Model for Microsurgery and Supermicrosurgery. Plast Reconstr Surg Glob Open 6:20–22. https://doi.org/10.1097/GOX.0000000000001695
Banda CH, Mitsui K, Ishiura R, Danno K, Narushima M (2020) A supermicrosurgery pig foot training model for practice of lymphaticovenular anastomosis. Microsurgery 40:91–92. https://doi.org/10.1002/micr.30521
Hayashi K, Hattori Y, Yii Chia DS, Sakamoto S, Marei A, Doi K (2018) A supermicrosurgery training model using the chicken mid and lower wing. J Plast Reconstr Aesthet Surg 71:943–945. https://doi.org/10.1016/j.bjps.2018.02.011
Albano NJ, Zeng W, Lin C, Uselmann AJ, Eliceiri KW, Poore SO (2021) Augmentation of Chicken Thigh Model with Fluorescence Imaging Allows for Real-Time, High Fidelity Assessment in Supermicrosurgery Training. J Reconstr Microsurg 37:514–518. https://doi.org/10.1055/s-0040-1722184
Lahiri A, Muttath SS, Yusoff SK, Chong AK (2020) Maintaining Effective Microsurgery Training with Reduced Utilisation of Live Rats. J Hand Surg Asian Pac 25:206–213. https://doi.org/10.1142/S2424835520500241
Prunières GJ-C, Taleb C, Hendriks S, Miyamoto H, Kuroshima N, Liverneaux PA et al (2014) Use of the Konnyaku Shirataki noodle as a low fidelity simulation training model for microvascular surgery in the operating theatre. Chir Main 33:106–11. https://doi.org/10.1016/J.MAIN.2013.12.003
Shimizu T, Yoshida A, Omokawa S, Tanaka Y (2019) A microsurgery training model using konjac flour noodles. Microsurgery 39:775–776. https://doi.org/10.1002/micr.30463
Dumont L, Hubert T, Guerreschi P (2011) La « double horloge » ou comment apprendre la microchirurgie sans animal The ‘“ double clock ”’ or how to learn microsurgery without animal. Annales de Chirurgie Plastique Esthetique 56:555–557. https://doi.org/10.1016/j.anplas.2011.08.014
Mehta A, Li PS (2013) Male infertility microsurgical training. Asian J Androl 15:61–66. https://doi.org/10.1038/aja.2012.86
Atlan M, Lellouch AG, Legagneux J, Chaouat M, Masquelet AC, Letourneur D (2018) A New Synthetic Model for Microvascular Anastomosis Training? A Randomized Comparative Study Between Silicone and Polyvinyl Alcohol Gelatin Tubes. J Surg Educ 75:182–187. https://doi.org/10.1016/j.jsurg.2017.06.008
Chan WY, Figus A, Ekwobi C, Srinivasan JR, Ramakrishnan VV (2010) The, “round-the-clock” training model for assessment and warm up of microsurgical skills: A validation study. J Plast Reconstr Aesthet Surg 63:1323–8. https://doi.org/10.1016/j.bjps.2009.06.027
Gunasagaran J, Rasid RJ, Mappiare S, Devarajooh C, Ahmad TS (2018) Microgrids: A model for basic microsurgery skills training. Malays Orthop J 12:37–41. https://doi.org/10.5704/MOJ.1807.007
Ince B, Yildirim MEC, Dadaci M (2019) A Low-Cost, Easily Accessible Simulation Model for Microsurgery Training. World J Plast Surg 8:265–6. https://doi.org/10.29252/wjps.8.2.265
Curran TA, Eves S, Williams GJ, Troisi L, Nicolaou M (2019) A simple, inexpensive and non-microscope based model for microsurgical training. J Plast Reconstr Aesthet Surg 72:1576–1606. https://doi.org/10.1016/j.bjps.2019.05.010
Kligman BE, Haddock NT, Garfein ES, Levine JP (2010) Microsurgery Trainer with Quantitativa Feedback: A Novel Training Tool for Microvascular Anastomosis and Suggested Training Exercise. Plast Reconstr Surg 126:328–330. https://doi.org/10.1097/PRS.0b013e3181f63fa4
Capkin S, Cavit A, Kaleli T (2018) Microsurgery training with smartphone. Handchir Mikrochir Plast Chir 50:443–445. https://doi.org/10.1055/a-0661-6015
Dos Reis JMC, Teixeira RKC, Dos SDR, Calvo FC, De Araújo NP, De Corrêa Junior WJP et al (2021) Novel Porcine Kidney-Based Microsurgery Training Model for Developing Basic to Advanced Microsurgical Skills. J Reconstr Microsurg 37:119–123. https://doi.org/10.1055/s-0040-1714428
Zeng W, Gunderson KA, Sanchez RJ, Albano NJ, Nkana ZH, Thadikonda KM et al (2021) The Blue-Blood Porcine Chest Wall: A Novel Microsurgery Training Simulator for Internal Mammary Vessel Dissection and Anastomosis. J Reconstr Microsurg 37:353–356. https://doi.org/10.1055/s-0040-1716859
Grossman LB, Komatsu DE, Badalamente MA, Braunstein AM, Hurst LC (2016) Microsurgical Simulation Exercise for Surgical Training. J Surg Educ 7:2456–2462. https://doi.org/10.1016/j.jsurg.2015.09.003
Karakawa R, Yoshimatsu H, Nakatsukasa S, Iida T (2017) A new method for microsurgery training using a smartphone and a laptop computer. Microsurgery 38:124–125. https://doi.org/10.1002/micr.30241
Karakawa R, Yoshimatsu H, Yano T, Sawaizumi M (2018) Microsurgery training using Apple iPad Pro. Microsurgery 38:926–927. https://doi.org/10.1002/micr.30384
Allouni A, Amer T, Ismail M, Ismail T (2016) The Human Umbilical Cord: a Model for Microsurgical Training. J Hand Microsurg 06:111–112. https://doi.org/10.1007/s12593-014-0142-6
Ishii N, Kiuchi T, Oji T, Kishi K (2016) Microsurgical training using reusable human vessels from discarded tissues in lymph node dissection. Arch Plast Surg 43:595–598. https://doi.org/10.5999/aps.2016.43.6.595
Achar RAN, Lozano PAM, Achar BN, Pereira Filho GV, Achar E (2011) Experimental model for learning in vascular surgery and microsurgery: esophagus and trachea of chicken. Acta Cir Bras 26:101–106. https://doi.org/10.1590/S0102-86502011000200005
Jeong H, Moon M, Kim H, Lee H, Yi S (2013) Microsurgical Training With Fresh Chicken Legs. Ann Plast Surg 70:57–61. https://doi.org/10.1097/SAP.0b013e31822f9931
Allan J, Dusseldorp J, Rabey NG, Malata CM, Goltsman D, Phoon AF (2015) Infrared Evaluation of The Heat-Sink Bipolar Diathermy Dissection Technique. J Plast Reconstr Aesthet Surg 68(8):1145–1151. https://doi.org/10.1016/j.bjps.2015.04.025
Malik MM, Hachach-Haram N, Tahir M, Al-Musabi M, Masud D, Mohanna PN (2017) Acquisition of basic microsurgery skills using home-based simulation training: A randomised control study. J Plast Reconstr Aesthet Surg 70:478–486. https://doi.org/10.1016/j.bjps.2016.12.011
Ramachandran S, Chui CHK, Tan BK (2013) The chicken aorta as a simulation-training model for microvascular surgery training. Arch Plast Surg 40(04):327–329. https://doi.org/10.5999/aps.2013.40.4.327
Cunico C, Benjamim ÃA, Brum S, Robes R, Freitas S (2016) Surgical Technique of Hemi-Face Transplant : A New Model of. Training 27:2013–2016. https://doi.org/10.1097/SCS.0000000000002449
Aurich LA, Silva Junior LFMD, Monteiro FMDR, Ottoni AN, Jung GS, Ramina R (2014) Microsurgical training model with nonliving swine head. Alternative for neurosurgical education. Acta Cir Bras 29:405–409. https://doi.org/10.1590/S0102-86502014000600010
Maluf Junior I, Silva ABDD, Groth AK, Lopes MAC, Kurogi AS, Freitas RDS, Tomasich FDS (2014) Modelo experimental alternativo para treinamento em microcirurgia. Rev Col Bras Cir 41:72–74. https://doi.org/10.1590/S0100-69912014000100014
Ghanem AM, Al Omran Y, Shatta B, Kim E, Myers S (2015) Anastomosis Lapse Index (ALI): A Validated End Product Assessment Tool for Simulation Microsurgery Training. J Reconstr Microsurg 32:233–241. https://doi.org/10.1055/s-0035-1568157
Kim E, Singh M, Akelina Y, Shurey S, Myers SR, Ghanem AM (2016) Effect of microvascular anastomosis technique on end product outcome in simulated training: a prospective blinded randomized controlled trial. J Reconstr Microsurg 32(7):556–561. https://doi.org/10.1055/s-0036-1584218
Safi AF, Safi S, Tayeh M, Timmer M, Goldbrunner R, Kauke M (2018) A novel microsurgical anastomosis training model using gradually thawed cryopreserved microvessels of rat cadavers. J Cranio-Maxillofac Surg 46:1126–1131. https://doi.org/10.1016/j.jcms.2018.05.018
Pamuk Ç (2022) Microsurgical training with chicken wings: Could it be an option to increase experience for vascularized bone flaps? Injury 53:422–426. https://doi.org/10.1016/j.injury.2021.12.054
Rodriguez JR, Yañez R, Cifuentes I, Varas J, Dagnino B (2016) Microsurgery workout: a novel simulation training curriculum based on nonliving models. Plast Reconstr surg 138(4):739e–747e. https://doi.org/10.1097/PRS.0000000000002456
Cigna E, Bistoni G, Trignano E, Tortorelli G, Spalvieri C, Scuderi N (2010) Microsurgical teaching: our experience. J Plast Reconstr Aesthet Surg 63:e529–e531. https://doi.org/10.1016/j.bjps.2009.10.011
Volovici V, Dammers R, Lawton MT, Dirven CMF, Ketelaar T, Lanzino G et al (2019) The Flower Petal Training System in Microsurgery: Validation of a Training Model Using a Randomized Controlled Trial. Ann Plast Surg 83:697–701. https://doi.org/10.1097/SAP.0000000000001914
Pafitanis G, Serrar Y, Raveendran M, Ghanem A, Myers S (2017) The chicken thigh adductor profundus free muscle flap: A novel validated non-living microsurgery simulation training model. Arch Plast Surg 44:293–300. https://doi.org/10.5999/aps.2017.44.4.293
Masud D, Haram N, Moustaki M, Chow W, Saour S, Mohanna PN (2017) Microsurgery simulation training system and set up: An essential system to complement every training programme. J Plast Reconstr Aesthet Surg 70:893–900. https://doi.org/10.1016/J.BJPS.2017.03.009
Trignano E, Fallico N, Zingone G, Dessy LA, Campus GV (2017) Microsurgical training with the three-step approach. J Reconstr Microsurg 33(2):87–91. https://doi.org/10.1055/s-0036-1592428
Oltean M, Sassu P, Hellstrom M, Axelsson P, Ewaldsson L, Nilsson A et al (2016) The microsurgical training programme in Gothenburg, Sweden: early experiences. J Plast Surg Hand Surg 51:1–6. https://doi.org/10.1080/2000656X.2016.1213735
Juratli MA, Becker F, Palmes D, Stöppeler S, Bahde R, Kebschull L et al (2021) Microsurgical training course for clinicians and scientists: a 10-year experience at the Münster University Hospital. BMC Med Educ 21:1–10. https://doi.org/10.1186/s12909-021-02737-1
Shulzhenko NO, Zeng W, Albano NJ, Lyon SM, Wieland AM, Mahajan AY et al (2020) Multispecialty Microsurgical Course Utilizing the Blue-Blood Chicken Thigh Model Significantly Improves Resident Comfort, Confidence, and Attitudes in Multiple Domains. J Reconstr Microsurg 36:142–150. https://doi.org/10.1055/s-0039-1700523
Yang Y, Ding M, Gong H, Hanken H, Zhao J, Tian L (2022) Portable fluid circuit device containing printed silicone microvessels as a training aid for arterial microanastomosis. Int J Oral Maxillofac Surg 51:1022–1026. https://doi.org/10.1016/j.ijom.2021.12.001
Schoeff S, Hernandez B, Robinson DJ, Jameson MJ, Shonka DC (2017) Microvascular anastomosis simulation using a chicken thigh model: Interval versus massed training. Laryngoscope 127:2490–2494. https://doi.org/10.1002/lary.26586
Creighton FX, Feng AL, Goyal N, Emerick K, Deschler D (2017) Chicken thigh microvascular training model improves resident surgical skills. Laryngoscope Investig Otolaryngol 2:471–474. https://doi.org/10.1002/lio2.94
Hino A, Batjer HH, Schackert G, Hashimoto N, Kobayashi S, Hongo K et al (2003) Training in microvascular surgery using a chicken wing artery. Neurosurgery 52:1495–1498. https://doi.org/10.1227/01.NEU.0000065174.83840.62
Couceiro J, Castro R, Tien H, Ozyurekoglu T (2015) Step by step: Microsurgical training method combining two nonliving animal models. J Visualized Exp 99:e52625. https://doi.org/10.3791/52625
Olabe J, Olabe J (2009) Microsurgical training on an in vitro chicken wing infusion model. Surg Neurol 72:695–699. https://doi.org/10.1016/j.surneu.2008.12.008
Jusue-Torres I, Sivakanthan S, Pinheiro-Neto CD, Gardner PA, Snyderman CH, Fernandez-Miranda JC (2013) Chicken wing training model for endoscopic microsurgery. J Neurol Surg B Skull Base 74:286–291. https://doi.org/10.1055/s-0033-1348026
Kaplan DJ, Vaz-Guimaraes F, Fernandez-Miranda JC, Snyderman CH (2015) Validation of a chicken wing training model for endoscopic microsurgical dissection. Laryngoscope 125:571–576. https://doi.org/10.1002/lary.24977
Hong JW, Kim YS, Lee WJ, Hong HJ, Roh TS, Song SY (2010) Evaluation of the efficacy of microsurgical practice through time factor added protocol: Microsurgical training using nonvital material. J Craniofac Surg 21:876–881. https://doi.org/10.1097/SCS.0b013e3181d7f2c7
Nam SM, Shin HS, Kim YB, Park ES, Choi CY (2013) Microsurgical training with porcine thigh infusion model. J Reconstr Microsurg 29:303–306. https://doi.org/10.1055/s-0033-1333623
Schoffl H, Hager D, Hinterdorfer C, Dunst KM, Froschauer S, Steiner W et al (2006) Pulsatile perfused porcine coronary arteries for microvascular training. Ann Plast Surg 57:213–216. https://doi.org/10.1097/01.sap.0000215248.70308.ae
Erel E, Aiyenibe B, Butler PEM (2003) Microsurgery simulators in virtual reality: Review. Microsurgery 23:147–152. https://doi.org/10.1002/micr.10106
Phoon AF, Gumley GJ, Rtshiladze MA (2010) Microsurgical Training Using a Pulsatile Membrane Pump and Chicken Thigh: A New, Realistic, Practical, Nonliving Educational Model. Plast Reconstr Surg 126:278e-e279. https://doi.org/10.1097/PRS.0b013e3181ef82e2
Zucal I, Feder AL, Kyaw T, Khin S, Heidekrueger PI, Prantl L et al (2022) An Innovative Simulation Model for Microvascular Training. Plast Reconstr Surg 150:189E-193E. https://doi.org/10.1097/PRS.0000000000009209
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
The study conceptualization and design were performed by Francesca Toia and Mara Franza. The literature search and data analysis were performed by Francesco Giuseppe Incandela, Emanuele Cammarata, Giorgio Romano, Luca Cicero, Giovanni Cassata, Roberta Cirincione. The first draft of the manuscript was written by Mara Franza. and all authors commented on previous versions of the manuscript. Francesca Toia and Salvatore Buscemi revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
No ethical approval, consent to participate or consent to publish was required for this review article.
Competing interests
Mara Franza, Salvatore Buscemi, Francesco Giuseppe Incandela, Emanuele Cammarata, Giorgio Romano, Luca Cicero, Giovanni Cassata, Roberta Cirincione, Francesca Toia declare no competing interests.
Additional information
Editor: Paolo Persichetti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Franza, M., Buscemi, S., Incandela, F.G. et al. Microsurgical training on non-living models: a systematic literature review. Eur J Plast Surg 47, 41 (2024). https://doi.org/10.1007/s00238-024-02184-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00238-024-02184-3