Abstract
Background
Numerous papers have been published evaluating the clinical benefits of robot-assisted microsurgery. In this study, we describe the incorporation of the MUSA-2 robot (Microsure, Eindhoven, The Netherlands) into a plastic surgery unit outside of its development centre. We aimed to assess robot implementation timeframes, areas of future development, and key learning points for centres considering the establishment of a robot-assisted microsurgery service.
Methods
We identified 12 female patients with upper limb lymphoedema secondary to breast cancer treatment, who consented to have a robotic-assisted lymphaticovenous anastomosis (LVA) from September 2022 to March 2023. All patients had at least one robot-assisted LVA. Post operatively, a surgical evaluation of the robot’s performance and of the surgeon’s intraoperative workload was measured. Each patient completed a postoperative Likert scale, which measured their surgical experience.
Results
The mean robot time per case was 60.25 min. The mean time taken for the first robot-assisted anastomosis was 32 min. The second robot anastomoses was 30% faster than the first, taking a mean of 22.5 min. The average anastomosis had 4.5 sutures placed robotically. Initial mean scores in the workload survey completed by the surgeon were highest for frustration and effort, both reduced with increasing volume of cases. In 91% of cases, physical intraoperative discomfort was reported by the surgeon but completely resolved following repositioning.
Conclusions
The current technology can be readily incorporated into a microsurgical unit. We developed four key learning points from the implementation of robot-assisted LVA in our microsurgical unit.
Level of evidence: Level IV, risk/prognostic study
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the development of microsurgery in the 1960s, it has become a reliable and replicable technique [1], enabling the reconstruction of complex oncological and traumatic defects in the breast, extremities, and head and neck [2,3,4]. Lymphaticovenous anastomosis (LVA) is an established supermicrosurgical technique that is recognised as an effective and physiological treatment for lymphoedema [5,6,7]. One important factor for treatment outcome is the patency of the anastomosis where accurately placed sutures are essential. There is little room for error, or the physiological tremor of the human hand [8]. Robot-assisted microsurgery offers many advantages, and it may improve the surgeon’s precision and may contribute to technological advancement within the field of microsurgery [9]. Robotic surgery can overcome unfavourable ergonomics and reduce the intraoperative demands on the surgeon [10]. Robotic limbs are not affected by tiredness, tremor, or concentration.
Currently, robotic surgery is used across a spectrum of gastrointestinal, urologic, and gynaecological procedures [11,12,13]. The da Vinci Surgical System (Intuitive Surgical Inc, Sunnyvale, CA) and Zeus Robotic Surgery Systems (Computer Motion, Goleta, CA) have proven benefits to both the surgeon and patient [14,15,16,17,18]. The da Vinci system, which is the most widely used robotic system, does not offer sufficiently high levels of magnification for microsurgical procedures, and its instruments are too cumbersome for microsurgical anastomoses [18]. In 2014, the Microsure Robot or MUSA-2 robot (Microsure, Eindhoven, The Netherlands) was developed in a collaboration between microsurgeons and engineers [19]. This system, which is the world’s first microsurgical robot with CE certification, confers the traditional advantages of the robotic surgery, such as motion scaling and tremor filtration but is compatible with pre-existing microscopes and micro-instruments. van Mulken et al. have shown, using the MUSA-2 robot, that robotic-assisted anastomoses had a steeper learning curve than hand-sewn anastomoses. The MicroSurgical Robot Research Group has proceeded to show, using 1-year follow-up data, that the MUSA-2 robot could be used, with comparable results, to hand-sewn LVA techniques for a cohort of lymphoedema patients [20, 21].
Uppsala University Hospital, Uppsala, Sweden, was chosen as the first site outside of the developing centre in The Netherlands to test the MUSA-2 robot in clinical practice. In this paper, we will describe the different steps for implementation of the MUSA-2 robot into our lymphoedema service. The data from the first twelve patients to have robotic-assisted LVA have been analysed and assessed according to a pre-implementation defined research protocol. The aim of this study was to define timeframes, areas of development, and key learning points for future centres considering implementation of a robot-assisted microsurgery service.
Methods
Study population
All patients with lymphoedema attending the Uppsala University Hospital lymphoedema clinic in 2023 were assessed according to a standardised protocol for lymphoedema. The protocol consisted of an assessment by a lymph-therapist, a plastic surgeon specialising in lymphoedema, and a clinical nurse specialist. Objective measurements of the limb were performed to assess for impedance, function, and volume, and indocyanine green (ICG) with near-infrared fluorescence was used to assess patient suitability for LVA. Based on the outcome of the patient’s assessment, patients were offered an LVA. The indications for surgery were upper limb lymphoedema in the presence of distally patent lymphatic vessels without clinical evidence of the sequalae of chronic upper limb lymphoedema. The patients must have had no improvement of lymphoedema symptoms after completing a minimum of 6 months wearing an optimised compression garment. If the patients were agreeable to the procedure, they were asked if they would consent to have the operation performed with robotic assistance or not. All eligible patients were operated on by a single surgeon (MM) with over 10 years of experience in microsurgery. Patients were operated on using the MUSA-2 between September 2022 and March 2023.
Data collection and analysis
Patient demographics and surgical variables such as operative time, number of anastomoses, anastomosis time, and number of sutures were recorded in a prospective database. Each patient completed a postoperative Likert scale and scored from 0 to 10, measuring their comfort, satisfaction with the surgical experience, and whether they would recommend this form of surgery. A surgical evaluation was performed by the operating surgeon. In the immediate post operative period. The number of adverse events under the categories of ‘planning and dexterity’, ‘visuospatial ability’, and ‘operative flow’ was recorded for each surgical procedure. A survey of the intraoperative workload experienced by the primary surgeon was assessed using a Likert scale, scored from 0 to 20, with 20 representing maximum demand.
Data analysis was performed in Microsoft Excel. Categorical data were described using counts and percentages. Due to the small numbers in the study, numerical data were described using both medians with ranges and means with standard deviations (SDs). The research protocol was approved by the Medical Research and Ethics Committee, with registration number of the ethical approval number DNr 2019–05040.
Robot preparation and installation
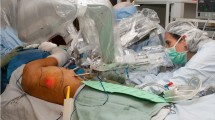
The robot was installed in the OR, either the night before surgery or in the morning before patient arrival. The master manipulator under the control of the primary surgeon was mounted on the surgical table, parallel to where the affected limb would be positioned. The theatre equipment and MUSA-2 were positioned in the operating room in a similar manner as described by van Mulken in a clinical trial. The standard surgical microscope (Zeiss Opmi Pentero 900; Carl Zeiss Meditec AG, Jena, Germany) was used which was positioned beside the patient’s contralateral shoulder (Fig. 1).
The setup in a robot-assisted LVA procedure of the right arm under local anaesthesia. The patient is in a supine position with the right arm abducted. The microscope is positioned beside the contralateral shoulder reaching into the surgical field and allowing the positioning of the robotic system at the ipsilateral side
Patient preparation and sterile draping
For the procedures performed under general anaesthesia (GA), the patient was intubated prior to any draping. Once the patient was anesthetised, their arm was positioned on the arm-table. The height of the robot ring holding the instruments was adjusted to the optimal position for the suturing of the anastomosis. The robot ring could then be moved to the side. The whole arm was then washed and draped in a standard fashion. Both the microscope and all parts of the robot were then draped with custom drapes.
For the two procedures performed using local anaesthesia (LA), the draping of the microscope and robot was done before the patient came into the OR. The patient’s arm was washed while they were sitting up, and they could then place the arm onto the arm table, which was covered with sterile drapes.
The robotic arms were loaded with standard microsurgical instruments just before the suturing of the anastomosis, a needle holder B-15–8.2 and dilator FRS-15 RM-8d.1 from S&T (S&T AG, Neuhausen, Switzerland).
Surgical procedure
A transverse skin incision was made at the site of LVA. Intraoperative fluorescent light from the microscope was used to find the lymphatics intraoperatively. Lumen size was measured with a specific supramicrosurgery ruler with lines of different thickness defining the size. A 7.0 monofilament suture was positioned in the lumen to stent the vessels open prior to anastomosis (Fig. 2). The operating field was covered by a moist gauze to protect the field while the robot was positioned in place (Fig. 3). LVAs were to be performed using robotic assistance in a standard end-to-end fashion using either 11–0 or 12–0 Ethilon (Ethicon, Johnson & Johnson, Bridgewater, NJ). The stenting suture was removed before placing the last two stitches. Patency of the LVA was assessed intraoperatively by the passage of ICG from the lymph vessel into the venule (Fig. 4).
All patients had at least one complete robot-assisted anastomosis. Where clinically indicated, patients had a second anastomosis which was performed either by hand-sewn techniques or using robot assistance. This was determined by surgeon preference. The primary surgeon was assisted by a plastic surgeon with microsurgical experience who sat opposite the primary surgeon. The main roles of the assistant were to maintain an optimal surgical field, to cut sutures after the knot had been tied, and also to pull the suture thread to a desirable length after the robot had passed the suture through the lymphatic vessel and vein. The robot can be used by one operating surgeon without modification to the equipment. The only ergonomic modification is that the single surgeon would manually cut their own suture after the knot has been tied using standard microsurgical instruments. The patients were discharged home the same day. The patients returned to the outpatient clinic after 2 weeks for a wound check, suture removal, and commencement of compression of the limb according to the standardised protocol. Patients were followed up as per protocol in the lymphoedema clinic at 1 month, 6 months, and 1 year.
Results
Twelve patients were recruited for this study, all of whom were female with a history of breast cancer and lymphoedema of either upper limb, secondary to their cancer treatment. Patient characteristics are summarised in Table 1.
The evaluation of the surgical procedure by the operating surgeon is summarised in Table 2. There were no adverse events involving catching the posterior wall of the lumen with the suture or inadvertent knot formation along the suture length. The most frequent event, which occurred in 6 cases, was loss of microscope focus. The robot and microscope are in a fixed position. However, during the suturing process of the anastomosis, the lymphatic vessel or vein is often mobilised. During supermicrosurgical anastomosis, these small movements can result in the lumen moving out of focus for the surgeon.
The total surgery time for each surgical case is outlined in Fig. 5. This was measured as the duration in minutes from the first incision to the application of dressings. The mean total surgery time was 212 min. The total time that the robot was used during each case is shown in Fig. 6. The mean robot time per case was 60.25 min. In the 12 cases, the mean time taken for the first robot-assisted anastomosis was 32 min. In cases where a second anastomosis was performed robotically, the mean robot time for both anastomoses was 51.5 min (n = 4). The second robot anastomoses took a mean time of 22.5 min. The second robot anastomosis was on average 30% faster than the first. Patients B, C, H, and I had their subsequent anastomoses performed using hand-sewn technique. In cases where the second anastomosis was performed manually, it took a mean of 15.5 min to perform (n = 4). For robotically performed anastomoses, the mean number of sutures placed was 4.5. The mean size of the venous lumen in the robot anastomoses was 0.38 mm, and the mean lymphatic vessel lumen was 0.49 mm. For hand-sewn anastomoses, the mean size of the vein lumen was 0.54 mm and the mean lumen of the lymphatic vessel was 0.32 mm.
Total surgical time, measured in minutes on the y-axis. Patients are listed on the x-axis. For patients who had one anastomosis, the bar is coloured black. Patients who had two anastomoses are coloured in blue. Patients who had three anastomoses are coloured red. Patient C had three anastomoses performed, but two of these were hand-sewn. Patients K and L had their procedure performed under LA. Patients A to J had their procedure performed under GA
The patient satisfaction scores were high in the post operative period. The exception is patient ‘G’, who had pain in her shoulder and arm after surgery. This was resolved without long-term sequalae. The patient satisfaction scores were higher in the patients who had GA 9.5 versus 8.4 in the LA group. The workload data is presented in Table 3.
Discussion
We believe that we are only at the beginning of what robotic surgery can do. The current study aims to contribute to this journey towards the future of microsurgery by defining key learning points. While other published papers evaluate the clinical benefits of robot-assisted microsurgery [20], the aim of the current study is to assess implementation timeframes, areas of development, and key learning points for future centres considering the establishment of a robot-assisted microsurgery service. The current assessment and training of microsurgical skill is based on subjective observation; we aimed to make an objective assessment of robot-assisted microsurgical technique [22].
Learning point 1
Familiarise the plastic surgery team, as a whole, with the robot. Effective familiarisation involves efficient robot installation and draping, but also how the robot functions during surgery.
It takes considerable conviction to trial a new technology while pre-existing techniques, such as hand-sewn LVA techniques, have proven benefits for the patient [23,24,25]. The MUSA robot system was brought to the microsurgical laboratory in Uppsala, 1 year in advance of the current study’s commencement. The senior surgeon, MM, did 3 days of training with Microsure and refresher training before we started the study. The robot being on site for 1 year allowed the plastic surgery team and the OR staff to understand and avoid common errors with the robot. The plastic surgery research nurse involved in the study was also the operating room scrub nurse for the robot-assisted cases. Initially, the installation of the robot was done in the OR, the night before surgery. As we became more confident, both the robot installation and sterile draping of the robot were done on the morning of surgery. The time taken to drape the robot ranged from 12 to 35 min. The time taken to drape by the OR nurse became shorter with practice. The implementation of the robot also forced us to rethink our standard practice of performing LVA under LA.
Learning point 2
Plan to perform the first robot procedures under general anaesthesia—in order for you to allow extra time for adjustments and procedural re-arrangements.
The first ten patients in the study had their surgery under GA as the procedure, and setting up of the instruments was anticipated to take longer, despite the standard LVAs at the department being normally performed under LA. It has been shown that surgical performance improves with experience [26] and simulated experience [27]. As a result, it was an expected finding that the surgical performance improved with increasing exposure to the robot. The mean total operation time in cases where two robot LVAs were performed was 216 min. This included the time taken to find the desired vessels, set up of anastomosis, positioning of the robot, suturing of the LVAs, and closure of the LVA sites. The mean time for the first robot anastomosis was 32 min. However, in cases where there was a second anastomosis performed robotically, it took a mean of 22.5 min. The second anastomosis was 30% faster than the first. While speed is not the only measurement of anastomotic quality, these data suggest that the time required to perform a robot anastomosis decreases with practice. It must also be considered that motion scaling, by its very nature, increases precision, and to do that, it slows down movement [28]. Our final two patients had their procedure under LA, lidocaine with 1% adrenaline, without encountering any problems. From this, we conclude that performing robot-assisted LVA under LA is feasible. However, performing the initial surgeries under GA allowed us to plan the implementation of the robot while reducing potential stress on the OR staff. It also allowed us to ‘trouble shoot’ any intraoperative difficulties encountered without the patient losing confidence in the procedure.
Learning point 3
Robotic surgery already offers many advantages in microsurgery, but there are a number of constraints that even an experienced microsurgeon must adapt to. Future generations of microsurgical robots must address these issues before robotic-assisted surgery becomes ubiquitous.
As with all microsurgery, the set up of the operative field is key. The current version of the MUSA robot used in this study has fixed ‘robot arms’ connected to a scaffold, which have a more limited arc of rotation in comparison to the human hand. As such, pre-anastomosis setup became an even more important determinate of anastomotic success. Intraoperative decisions regarding the operative field and vessel preparation in advance of the anastomosis are similar to both hand-sewn and robot-assisted LVA. As a result, errors in judgement in the preparation stage were less common than in other parts of the anastomosis. An inappropriate operative field and insufficient vessel preparation occurred more than once in just 25% of cases. This issue will likely be resolved with the next generation of the MUSA robot as this will have free-ranging ‘robot arms’ which will significantly improve the arc of rotation of the robot limbs. The Symani surgical system (Medical Microinstruments (MMI), Calci, Italy) has flexible robot arms for robot-assisted microsurgery, and the early experience was published in January 2022 [28, 29].
Robot suturing is a new technique that the surgeon must learn and adapt to. This demands patience and application from the surgeon. During the anastomosis, the suture was broken in the robotic instruments at least once in 75% of cases. This is shown in the post operative workload survey that showed the highest mean score in frustration 15/20, followed by a mean effort score of 14.6/20. In this study, a learning curve as evidenced by a reduction in time taken to perform the robot-assisted LVA was not observed. However, reductions in surgeon-reported frustration and effort were both observed. This could also be improved by the addition of haptic feedback by future iterations of the MUSA robot.
This version of the MUSA robot has not yet been optimally designed for the comfort of the primary surgeon. Its use requires shoulder extension with elbow flexion to move the master manipulators (Figs. 7 and 8). To engage the robot, it also requires plantar and dorsiflexion of the foot pedal. This led to physical intraoperative discomfort attributed to the surgery in 91% of the cases. All of the pain was resolved with the repositioning of the surgeon. This will be addressed in future iteration of the MUSA robot.
Learning point 4
The relationship between the robot provider and surgeon is of paramount importance to the successful implementation of a robot.
The establishment of a microsurgical robot outside of the centre of development of the robot is entirely possible, but both the surgeon and robot provider must be engaged in the process. In our experience, the robot implementation required adjustments and servicing at different timepoints by the robot provider. A prerequisite for the development of a technical device is an honest and reciprocal relationship between both the surgeon and the developing company. This necessitated that the robot engineers being present during surgery and that the surgeon was open to constructive feedback from the engineers. This led to a working relationship that was entirely focussed on the improvement of the robot. All of our feedback has been taken into account, and we look forward to using future versions of the MUSA robot.
There is a growing number of reviews examining the current and potential future role of robotics in reconstructive microsurgery [30,31,32,33]. However, looking at the published trials of robotic-assisted microsurgery, there are some common difficulties with all of the systems. [19, 29, 34]. Establishment of a robot comes with financial costs of the system but also of the potential costs associated with single-use instruments and sterile drapes [29]. However, an integral part of our job, for our specialities’ advancement and our patient’s benefit, is to challenge established techniques with new techniques. Consequently, there is a price to be paid by the surgeon, in the form of time. The time taken to learn a new technique. The time taken to train the nursing staff. The time taken to prepare the OR. The time, patience, and motivation required to master a new technique in the OR. Our conclusion regarding the implementation of robot-assisted microsurgery is that there is an initial ‘big step’ but that this is overcome within a reasonable number of cases.
Limitations
To our knowledge, there are no published papers evaluating a surgical robot performance and its interaction with a surgeon. As a result, the scales used to assess performance had to be designed prior to the study commencement by the surgical team.
A number of patients had a hand-sewn LVA as well as a LVA performed robotically. While this paper is primarily about presenting our implementation strategies and reflections, this confounds our data and makes it harder to compare the robot performance. We chose to perform the optimum number of anastomoses to achieve the best outcome for the patient within the confines of equitable theatre access to all of our patients. This resulted in some of the LVAs being performed using a hand-sewn technique.
Conclusions
We hope that this study describes our experience of establishing a robotic microsurgical service and relevant learning points. The current technology can be readily incorporated into a microsurgical unit. Consistency in the staff involved enables a steep learning curve both for setup and the surgery itself. For an experienced surgeon, a robot only addressing the anastomoses per se is likely to be of less benefit than it is to an inexperienced surgeon. There are likely different learning curves and different levels of potential gain depending on the surgeon’s baseline. Future iterations of the MUSA robot are expected to overcome the challenges encountered at this first implementation trial.
“What would life be if we had no courage to attempt anything?” – Vincent Van Gogh.
Data Availability
The data used in this study is not availabe to the general public. It may be available upon request to the corresponding author.
References
Tamai S (2009) History of microsurgery. Plast Reconst Surg 124:e282–e294
Xiong L, Gazyakan E, Kremer T, Hernekamp FJ, Harhaus L, Saint-Cyr M et al (2016) Free flaps for reconstruction of soft tissue defects in lower extremity: a meta-analysis on microsurgical outcome and safety. Microsurgery. [cited 2023 Feb 8];36(6):511–24. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002/micr.30020. Accessed 8 Feb 2023
Khajuria A, Prokopenko M, Greenfield M, Smith O, Pusic AL, Mosahebi A (2019) A meta-analysis of clinical, patient-reported outcomes and cost of DIEP versus implant-based breast reconstruction. Plast Reconstr Surg Glob Open 7(10):E2486
Chorath K, Go B, Shinn JR, Mady LJ, Poonia S, Newman J et al (2021) Enhanced recovery after surgery for head and neck free flap reconstruction: a systematic review and meta-analysis. Oral Oncol. 113:105117
Koshima I, Inagawa K, Urushibara K, Moriguchi T (2000) Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg 16(6):437–442
Basta MN, Gao LL, Wu LC (2014) Operative treatment of peripheral lymphedema: a systematic meta-analysis of the efficacy and safety of lymphovenous microsurgery and tissue transplantation. Plast Reconstr Surg 133(4):905–913
Hong JP (Jp), Song S, Suh HSP (2018) Supermicrosurgery: principles and applications. J Surg Oncol. 118(5):832–9
Harwell RC, Ferguson RL (1983) Physiologic tremor and microsurgery. Microsurgery 4(3):187–192
Aitzetmüller MM, Klietz ML, Dermietzel AF, Hirsch T, Kückelhaus M (2022) Robotic-Assisted microsurgery and its future in plastic surgery. J Clin Med MDPI 11(12):3378
Lakhiani C, Fisher SM, Janhofer DE, Song DH (2018) Ergonomics in microsurgery. J Surg Oncol 118(5):840–844
Konstantinidis IT, Ituarte P, Woo Y, Warner SG, Melstrom K, Kim J et al (2020) Trends and outcomes of robotic surgery for gastrointestinal (GI) cancers in the USA: maintaining perioperative and oncologic safety. Surg Endosc 34(11):4932–4942
Sinha R, Sanjay M, Rupa B, Kumari S (2015) Robotic surgery in gynecology. J Min Access Surg. Medknow Publications 11:50–9
Pal RP, Koupparis AJ (2018) Expanding the indications of robotic surgery in urology: a systematic review of the literature. Arab J Urol. [cited 2023 Feb 9];16(3):270–84. Available from: https://www.tandfonline.com/action/journalInformation?journalCode=taju20. Accessed 9 Feb 2023
van der Hulst R, Sawor J, Bouvy N (2007) Microvascular anastomosis: is there a role for robotic surgery? J Plast Reconstr Aesthet Surg 60(1):101–102
Knight CG, Lorincz A, Cao A, Gidell K, Klein MD, Langenburg SE (2005) Computer-assisted, robot-enhanced open microsurgery in an animal model. J Laparoendosc Adv Surg Tech A 15(2):182–185
Dobbs TD, Cundy O, Samarendra H, Khan K, Whitaker IS (2017) A Systematic review of the role of robotics in plastic and reconstructive surgery - from inception to the future. Front Surg 4:66
Katz RD, Rosson GD, Taylor JA, Singh NK (2005) Robotics in microsurgery: use of a surgical robot to perform a free flap in a pig. Microsurgery 25(7):566–569
Taleb C, Nectoux E, Liverneaux PA (2008) Telemicrosurgery: a feasibility study in a rat model. Chir Main 27(2–3):104–108
Van Mulken TJM, Boymans CAEM, Schols RM, Cau R, Schoenmakers FBF, Hoekstra LT et al (2018) Preclinical experience using a new robotic system created for microsurgery. Plast Reconstr Surg 142(5):1367–1376
van Mulken TJM, Wolfs JAGN, Qiu SS, Scharmga AMJ, Schols RM, Spiekerman van Weezelenburg MA et al (2022) One-year outcomes of the first human trial on robot-assisted lymphaticovenous anastomosis for breast cancer–related lymphedema. Plast Reconstr Surg 149(1):151–61
Van Mulken TJM, Schols RM, Scharmga AMJ et al (2020) First-in-human robotic supermicrosurgery using a dedicated microsurgical robot for treating breast cancer-related lymphedema: a randomized pilot trial. Nat Commun 11(1):757
van Mulken TJM, Scharmga AMJ, Schols RM, Cau R, Jonis Y, Qiu SS et al (2020) The journey of creating the first dedicated platform for robot-assisted (super)microsurgery in reconstructive surgery. Eur J Plast Surg 43(1):1–6
Scaglioni MF, Fontein DBY, Arvanitakis M, Giovanoli P (2017) Systematic review of lymphovenous anastomosis (LVA) for the treatment of lymphedema. Microsurgery 37(8):947–953
Gupta N, Verhey EM et al (2021) Outcomes of lymphovenous anastomosis for upper extremity lymphedema: a systematic review. Plast Reconstr Surg Glob Open 9(8):e3770
Forte AJ, Sisti A, Huayllani MT, Boczar D, Cinotto G, Ciudad P et al (2020) Lymphaticovenular anastomosis for breast cancer-related upper extremity lymphedema: a literature review. Gland Surg 9(2):539–544
Maruthappu M, Gilbert BJ, El-Harasis MA, Nagendran M, McCulloch P, Duclos A et al (2015) The influence of volume and experience on individual surgical performance: a systematic review. Ann Surg 261(4):642–647
Beth Grossman L, Komatsu DE, Badalamente MA, Braunstein AM, Hurst LC (2016) Microsurgical simulation exercise for surgical training. J Surg Educ 73(1):116–120
Barbon C, Grünherz L, Uyulmaz S, Giovanoli P, Lindenblatt N (2022) Exploring the learning curve of a new robotic microsurgical system for microsurgery. JPRAS Open. [cited 2023 Apr 12];34:126–33. Available from: https://pubmed.ncbi.nlm.nih.gov/36304073/. Accessed 12 Apr 2023
Lindenblatt N, Grünherz L, Wang A, Gousopoulos E, Barbon C, Uyulmaz S et al (2022) Early experience using a new robotic microsurgical system for lymphatic surgery. Plast Reconstr Surg Glob Open. [cited 2023 Apr 12];10(1):E4013. Available from: https://journals.lww.com/prsgo/Fulltext/2022/01000/Early_Experience_Using_a_New_Robotic_Microsurgical.9.aspx. Accessed 12 Apr 2023
Dobbs TD, Cundy O, Samarendra H, Khan K, Whitaker IS (2017) A systematic review of the role of robotics in plastic and reconstructive surgery-from inception to the future. Front Surg [cited 2023 Apr 12];4. Available from: https://pubmed.ncbi.nlm.nih.gov/29188219/. Accessed 12 Apr 2023
Grünherz L, Gousopoulos E, Barbon C, Uyulmaz S, Giovanoli P, Lindenblatt N (2023) Robotics in plastic surgery. Chirurgie (Heidelberg, Germany). [cited 2023 Apr 12];94(4). Available from: https://pubmed.ncbi.nlm.nih.gov/36625922/. Accessed 12 Apr 2023
Saleh DB, Syed M, Kulendren D, Ramakrishnan V, Liverneaux PA (2015) Plastic and reconstructive robotic microsurgery--a review of current practices. Ann Chir Plast Esthet. [cited 2023 Apr 12];60(4):305–12. Available from: https://pubmed.ncbi.nlm.nih.gov/25896870/. Accessed 12 Apr 2023
Selber JC (2020) The robotic DIEP flap. Plast Reconstr Surg 145(2):340–343
Innocenti M (2022) Back to the future: robotic microsurgery. Arch Plast Surg 49(3):287–288
Funding
Open access funding provided by Uppsala University. The robot lease was funded by the Lundberg grant received by Maria Mani in 2019. Microsure supported the part-time salary of the research nurse participating in the trial. The other authors have no financial disclosures. The authors declare that no funds, grants, or other supports were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Helena Frieburg, Anna Nilsson and Michael S Mayr-Riedler. The first draft of the manuscript was written by Frank Reilly and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Uppsala University. The registration number of the ethical approval is DNr 2019–05040.
Consent for publication
The authors affirm that human research participants provided informed consent for the publication of all data used in this study.
Competing interests
Maria Mani is a member of the medical advisory board of Microsure since June 2022. The other authors have no financial disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reilly, F.O.F., Nilsson, A., Frieberg, H. et al. Implementation of robot-assisted lymphaticovenous anastomoses in a microsurgical unit. Eur J Plast Surg 47, 17 (2024). https://doi.org/10.1007/s00238-024-02163-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00238-024-02163-8