Abstract
Background
Our team developed a new patient-reported outcome measure to evaluate outcomes in patients having surgery for an acquired or congenital external ear anomaly. The aim of this study was to translate and culturally adapt the EAR-Q into Arabic, Chinese, French, and Spanish (Spain).
Methods
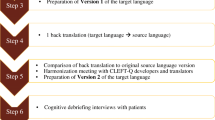
Translation and cultural adaptation guidelines set forth by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) were followed.
Results
The EAR-Q consists of 30 items in 2 scales measuring the look and feel of one’s ears in addition to 2 stand-alone items about scars and 1 item about hearing aids. Forward translations revealed no items, instructions, or response options that were difficult to translate into any of the languages. After back translation, the meaning of 6 (18%) items from the Arabic and Spanish translations, and 4 (12%) items from the Chinese and French translations, respectively, differed from the English version and required revision. Final translations were shown to 28 patients with an ear condition who took part in cognitive debriefing interviews. Participants were recruited from plastic surgery centers in Qatar, China, Spain, and an Ear Nose and Throat center in France. Mean age for participants was 10 years (5 to 18), 15 were male, and 50% had prominent ears (n = 14). Participants reported few difficulties with understanding the scales.
Conclusions
Translation and cultural adaptation of the EAR-Q resulted in 4 translations that maintained semantic, idiomatic, experiential, and conceptual equivalence to the original English version.
Level of evidence: Level III, risk/prognostic study.


Similar content being viewed by others
References
Kapp-Simon KA, Simon DJ, Kristovich S (1992) Self-perception, social skills, adjustment, and inhibition in young adolescents with craniofacial anomalies. Cleft Palate Craniofac J 29:352–356
Horlock N, Vögelin E, Bradbury ET, Grobbelaar AO, Gault DT (2005) Psychosocial outcome of patients after ear reconstruction: a retrospective study of 62 patients. Ann Plast Surg 54:517–524
Sheerin D, MacLeod M, Kusumakar V (1995) Psychosocial adjustment in children with port-wine stains and prominent ears. J Am Acad Child Adolesc Psychiatry 34:1637–1647
Baluch N, Nagata S, Park C et al (2014) Auricular reconstruction for microtia: a review of available methods. Plast Surg 22(1):39–43
Sarwer DB, Whitaker LA, Pertschuk MJ et al (1998) Body image concerns of reconstructive surgery patients: an underrecognized problem. Ann Plast Surg 40:403–407
Longmire NM, Wong Riff KWY, O'Hara JL, Aggarwala S, Allen GC, Bulstrode NW, Forrest CR, French BM, Goodacre TEE, Marucci D, Norris JH, Panchapakesan V, Piplani B, Pusic AL, Vercruysse HJ, Klassen AF (2017) Development of a new module of the FACE-Q for children and young adults with diverse conditions associated with visible and/or functional facial differences. Facial Plast Surg 33(5):499–508
Klassen AF, Longmire NM, Bulstrode NW, Fisher DM, Kasrai L, O'Hara J, Panchapakesan V, Pusic AL, Stewart K, Tsangaris E, Ziolkowski N, Wong Riff KWY (2018) Development of a new patient-reported outcome measure for ear conditions: The EAR-Q. Plast Reconstr Surg Glob Open 6(8):e1842
Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee-Lorenz A, Erikson P (2005) Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 8(2):94–104
2nd Congress of the International Society for Auricular Reconstruction. Retrieved from: http://www.isar.cc/wp-content/uploads/2018/01/ISAR-Final-Printed-Programme.pdf [July 10, 2019]
Tsangaris E, Wong Riff KWY, Vargas F, Aguilera MP, Alarcón MM, Cazalla AA, Thabane L, Thoma A, Klassen AF (2017) Translation and cultural adaptation of the CLEFT-Q for use in Colombia, Chile, and Spain. Health Qual Life Outcomes 15:228
Hunt SM, Bhopal R (2004) Self-report in clinical and epidemiological studies with non-English speakers: the challenge of language and culture. J Epidemiol Community Health 58(7):618–622
Willis GB (2015) Analysis of the cognitive interview in questionnaire design: understanding qualitative research. Oxford University Press, Toronto
Willis GB (2005) Cognitive interviewing: a tool for improving questionnaire design. Sage Publications, New York
Guillemin F, Bombardier C, Beaton D (1993) Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. J Clin Epidemiol 46:1417–1432
US Food and Drug Administration Guidance for Industry (2009) Patient-reported outcome measures: use in medical product development to support labeling claims. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Rockville
COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN). Research Projects. Retrieved from: https://www.cosmin.nl/research-publications/. Accessed July 27, 2019.
Aaronson N, Alonso J, Burnam A, Lohr KN, Patrick DL, Perrin E, Stein RE (2002) Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res 11(3):193–205
Patrick DL, Burke LB, Gwaltney CJ et al (2011) Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 1—eliciting concepts for a new PRO instrument. Value Health 14:967–977
Patrick DL, Burke LB, Gwaltney CJ et al (2011) Content validity—establishing and reporting the evidence in newly developed pa- tient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2—assessing respondent understanding. Value Health 14:978–988
Terwee CB, Prinsen CAC, Chiarotto A, Westerman MJ, Patrick DL, Alonso J, Bouter LM, de Vet HCW, Mokkink LB (2018) COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res 27(5):1159–1170
Acknowledgements
We would like to thank all the translators as well as Dr. Khalid El-Maaytah (Royal Medical Services, Jordan) and Ms. Sophia Zhao for their valuable contributions in developing the Arabic and Chinese translation of the EAR-Q, respectively. We would also like to thank the patients who participated in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors are grateful for grant funding provided by the Canadian Institutes for Health Research and the Plastic Surgery Foundation.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Hamilton Integrated Research Ethics Board (HiREB) along with the Research Ethics boards at each of the following participating hospitals: Ninth People’s Hospital Shanghai, China; McGill University, Montreal, Canada; Hospital Necker Enfants Malades, Paris, France; Hospital General Universitario Gregorio Maranon, Madrid, Spain; and Hamad Medical Corporation in Doha, Qatar.
Informed consent
All participants and/or their legal guardians (when appropriate) provided written informed consent or assent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsangaris, E., Riff, K.W.Y.W., Berenguer, B. et al. Translation and cultural adaptation of the EAR-Q into Arabic, Chinese, French and Spanish for use in an international field-test study. Eur J Plast Surg 43, 159–164 (2020). https://doi.org/10.1007/s00238-019-01585-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00238-019-01585-z