Abstract
Purpose
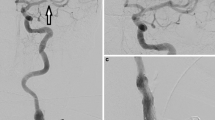
Double-layer design carotid stents have been cast in a negative light since several investigations reported high rates of in-stent occlusions, at least in the acute setting of tandem occlusions. CGuard is a new generation double-layered stent that was designed to prevent periinterventional embolic events. The aim of this study was to analyze the safety and efficacy of the CGuard in emergent CAS and for the acute treatment of tandem occlusions in comparison with the single-layer Carotid Wallstent (CWS) system.
Methods
All patients who underwent CAS with CGuard or CWS after intracranial mechanical thrombectomy (MT) between 11/2018 and 12/2022 were identified from our local thrombectomy registry. Clinical, interventional and neuroimaging data were analyzed. Patency of the stent was assessed within 72 h. Intracranial hemorrhage and modified Rankin score (mRS) at discharge were the main endpoints.
Results
In total, 86 stent procedures in 86 patients were included (CWS: 44, CGuard: 42). CGuard had a lower, but not statistically significant rate (p = 0.431) of in-stent occlusions (n = 2, 4.8%) when compared to the CWS (n = 4, 9.1%). Significant in-stent stenosis was found in one case in each group. There was no statistically significant difference in functional outcome at discharge between the two groups with a median mRS for CGuard of 2 (IQR:1–5) vs. CWS 3 (IQR:2–4).
Conclusion
In our series, the rate of in-stent occlusions after emergent CAS was lower with the dual-layer CGuard when compared to the monolayer CWS. Further data are needed to evaluate the potential benefit of the design in more detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mechanical thrombectomy (MT) has established itself as the best possible treatment in the setting of an acute ischemic stroke (AIS) [1]. Up to 15% of all patients with a large vessel occlusion (LVO) in the anterior circulation also have an extracranial occlusion or high-grade stenosis [2, 3]. Tandem occlusions (TO) are associated with an increased risk for intracranial hemorrhage (ICH) and have in general an unfavorable prognosis [4]. In a setting of TO, intracranial treatment is frequently performed first to hasten early brain reperfusion and then acute carotid artery stenting (aCAS) is carried out [5, 6]. In instances of acutely symptomatic thrombogenic sub-occlusive lesions affecting the extracranial internal carotid artery ICA, stenting is frequently employed to address the specific lesion and prevent the recurrence of thromboembolism in the intracranial circulation. Not treating the culprit lesion is associated with a potential risk of thrombus formation and stroke recurrence [7, 8]. Analyzed data from the German stroke registry, as well as the TITAN and ETIS registries demonstrated that recanalization of the ICA in TO leads to a significantly higher probability of successful reperfusion, better clinical outcome and significantly lower mortality [9, 10]. Acute CAS offers a one-stop treatment of the causative plaque and thus reduces the risk of reoccurrence [11]. However, such interventions are associated with the risk of in-stent thrombosis, ICH, and postprocedural ischemic events [4, 12]. The latter is attributed to the detachment of thrombus and plaque debris through the stent and occurs most likely when open-cell design [13] and not closed-cell design stents were used. Dual-layer/mesh stents (DLS) were designed in order to offer better plaque coverage and by this reducing periinterventional as well as secondary thromboembolic events. However, the great expectations placed on this new stent design were disappointed after several studies had reported high occlusion rates after the use of the dual-layer Casper-RX (Microvention, Tustin, CA, USA) in an emergent setting [14,15,16]. This discouraged interventionalists from implementing DLS in acute settings. Nevertheless, CGuard (InspireMD Inc., Tel Aviv, Israel) has a completely different design and has shown promising results in several multicentric trials in an elective setting [17, 18] whereas data on the use of the CGuard in acute CAS are very limited [19].
We aimed to compare the patency of the CGuard with the single-layer Carotid Wall-stent (CWS) in patients with acute symptomatic extracranial ICA occlusion or high-grade stenosis with or without TO, up to 72 h after the intervention.
Methods
Our prospective local thrombectomy registry was retrospectively analyzed for consecutive patients with AIS due to an acute symptomatic extracranial ICA occlusion or high-grade stenosis with or without TO, who received endovascular treatment between 11/2018 and 12/2022. In our comprehensive stroke center approximately 40% of MT patients are referred from other external hospitals and centers. Figure 1 represents a flowchart of the included patients from the comprehensive stroke center.
Inclusion criteria
-
Patients were referred for acute endovascular treatment and received emergent CAS using CGuard or CWS.
-
Diagnosis was immediately established after computed tomography (CT) or magnetic resonance imaging (MRI) by the neuroradiologist.
-
Patients showed a significant neurological deficit after undergoing the neurological examination per the National Institutes of Health Stroke Scale (NIHSS) score ≥ 4 before the intervention.
-
Premorbid modified Rankin Scale (mRS) score ≤ 3.
-
Achieved recanalization with a modified Thrombolysis in Cerebral Infarction (TICI) ≥ 2b.
-
Patients who received follow-up imaging of the brain and the cervical arteries using CT, CT angiography, MR or Doppler ultrasonography to evaluate stent patency, detect intracranial hemorrhage and Infarction within 72 h after the intervention or earlier until discharge.
When indicated, intravenous r-tPA (recombinant tissue-type plasminogen activator) was administrated to eligible patients prior to endovascular therapy.
Research was conducted according to the principles of the declaration of Helsinki.
Study device
The CGuard stent-system is a double-layered stent that consists of an inner open-cell nitinol stent and an outer closed-cell, single knitted polyethylene terephthalate (PET) mesh layer of 20 μm. It is a self-expandable stent delivered through a 6-F (2.0-mm) catheter [17]. The stent is available in sizes from 6 to 10 mm in diameter and lengths from 20 to 60 mm. The pore size of the mesh when the stent is fully deployed is 150 to 180 μm, with a free cell area of 16.25 mm2.
The CWS (Boston Scientific, Santa Clara, California) is a single-layer system consisting of cobalt-chromium alloy with closed-cell design and a free cell area of 1.09 mm2 [20]. Depending on stent size it is 5-F and 6-F sheath compatible.
The differences between both stent designs are highlighted in Fig. 2.
Illustration demonstrating the difference between the free cell size (white emphasized space) and pore size (grey emphasized space) between Wall-stent (A) and CGuard-stent (B). Free cell size offers flexibility and conformability during deployment, especially in torturous vessels. The pore size offers proper coverage of the underlying plaque
Endovascular procedures and peri-interventional antiplatelet/anticoagulation
All acute MT and CAS procedures were performed under general anesthesia via a transfemoral approach. Generally speaking, a short 8F-sheath was used and an 8F guiding catheter was placed in the proximal CCA with support of a 5F selective catheter. For passing the stenosis of the internal carotid artery or occlusion normally, a 0.014” microwire (Traxess, Microinvention, Aliso Viejo, CA, USA) was used. No distal protection devices were used in a setting of acute CAS. Further materials used were according to the judgment and experience of the neurointerventionalist. A retrograde approach (intracranial procedure before the CAS) was preferred and an antegrade approach was only chosen if the passage of the proximal occlusion was not possible otherwise. The indication for carotid artery stenting [with percutaneous transluminal angioplasty (PTA) as necessary] was given if a high-grade and hemodynamic stenosis remained after recanalizing the vessel, or if the risk of rapid re-occlusion was considered high due to the configuration of the lesion. Principally, all patients received initially intravenous heparin 3000 UI, and an additional 1000 IU for every additional hour during the intervention. Patients received i.v. acetylsalicylic acid ASA (500 mg) before stent-implantation. Per protocol after achieving intracranial recanalization and treating the culprit ICA lesion, an angiography of the intracranial circulation is performed to ensure that no new embolization to the intracranial circulation has occurred during the CAS. There were no constraints regarding the use of stent retrievers or aspiration maneuvers for MT of the intracranial occlusion if applicable, or different antiplatelet regimens and anticoagulants. In addition, there were no constraints regarding the succession of CAS with or without balloon angioplasty and MT. Periprocedural intravenous thrombolytic drugs were administered at the discretion of the treating neurologist and neuroradiologist.
Peri- interventional antiplatelet therapy
Within 24 h after MT and CAS patients underwent follow-up with cranial CT to exclude significant intracranial hemorrhage. If no contraindications were detected dual-antiplatelet therapy (DAPT) was started. Platelet function test was performed with Multiplate testing to identify partial/non-responders.
Outcome evaluation
NIHSS was evaluated at admission and discharge again by an experienced neurologist. To evaluate the infarct progression and whether intracranial hemorrhage is present, all patients received a CT or MRI within 24 h after the intervention. Intracranial hemorrhage was assessed according to the criteria of the European Cooperative Acute Stroke Study ECASS II [21]. The mRS was used as indicator of clinical outcome at discharge.
Statistical analysis
Baseline patient characteristics were analyzed using descriptive statistics. We performed chi-squared tests for categorical variables and two-sided t-tests for continuous variables. Data are shown as mean with standard deviation (SD) or median with interquartile range (IQR). All calculations were performed using SPSS software (.
Results
All patients with acute occlusions or high-grade stenosis of the extracranial ICA with or without middle cerebral artery (MCA) and/or intracranial ICA (carotid terminus) occlusions who were treated between November 2018 and December 2022 were identified from our stroke registry and were retrospectively analyzed. In this study, the first included CWS was used in November 2018 and the CGuard stent was first used in an emergent setting in November 2019 in our center. Three patients were excluded: one patient who underwent intra- and extracranial stenting, one patient who was treated with a CGuard stent after an acute in-stent-occlusion of another ICA stent, and one patient who was treated with a CWS for an acute dissection and with a CGuard after acute occlusion of the first stent. In addition, all patients with acute dissections who were treated with aspiration only or other stents/ flow diverters were not included in this study.
Overall, eighty-six patients were included: Forty-four patients were treated using the CWS and forty-two with the CGuard-stent. The underlying etiology of the high-grade stenosis of the ICA or occlusion was dissection in 5 patients (5.8%) and atherosclerosis in 81 patients (94.2%). The rates of the initial complete occlusions of the cervical ICA did not differ significantly between CWS- and CGuard-groups (59.1% versus 50%; p = 0.397). Balloon angioplasty prior to stenting was performed in both groups (40.9% vs. 35.7%, p = 0.620). Post-dilation was performed in both groups (22.7% vs. 19%, p = 0.537). All stents were patent on the final angiograms and no rest significant stenosis (> 50%) was detected after completion of the intervention. Mean age of patients in both groups was similar (CWS: 71.4, CGuard: 71.9) with a predominant number of male patients 70.5% and 69% respectively. Baseline median NIHSS score was 13 (IQR: 9–17) and 12 (IQR: 7–17) respectively with 45.5% and 47.6% of patients receiving IV tPA.
Baseline, demographic, interventional and imaging characteristics of the overall patient population are shown in Table 1.
The rate of acute in-stent occlusions (occlusions within 72 h after stenting) was lower in the CGuard group when compared to the CWS group without statistical significance (n = 2; 4.8% vs. n = 4; 9.1%, p = 0.431). Three of the patients with acute in-stent occlusions (2 CWS and 1 CGuard) had dissection as an underlying pathology for the initial ICA occlusion. These occlusions occurred in less than 24 h postprocedural. The rest of the in-stent occlusions occurred between 48 and 72 h and each of those patients received DAPT postprocedural. Each group had one case of in-stent stenosis. CGuard patients performed slightly better with a discharge mRS of 2 (IQR:1–5) vs. 3 (IQR:2–4), p = 0.285, again without statistical significance. 52.4% of the CGuard group n = 22 had favorable outcomes at discharge (mRS ≥ 2) vs. CWS group n = 15, 34.1%, p = 0.087.
Patients received between 3000 and 8000 IU of heparin depending on the duration of the intervention. There was a statistically significant difference with regard to antiplatelet regimen administered after deployment of the stents (p < 0.001): CWS group received primarily Clopidogrel/ Acetylsalicylic acid (ASA) (75%), while the CGuard-group received predominantly Ticagrelor/ASA (83.3%).
The subgroup analysis of antiplatelet regimen among CWS patients did not reveal any significant differences regarding in-stent-occlusions or –stenosis. The same applies to the CGuard patients. Platelet inhibition testing didn’t recognize non- responders or impaired response to CPG among patients with in-stent occlusions. Intracranial hemorrhage was observed more often in the CWS-group without any statistical significance (p = 0.652; 20.5% vs. 16.7%). In addition, no significant association between the antiplatelet regimens and intracranial hemorrhage was found. Different antiplatelet regimens and frequency of ICH are presented in Table 2.
Only 15/42 (35.7%) of the CGuard group and 18/44 (40.9%) of the CWS-group received follow-up imaging at 6 months in our center. All CGuard stents were patent without stenosis. However, 6/18 (33.3) of the CWS group presented with in-stent stenosis and the difference between both groups was statistically significant (p = 0.039). Half of the CWS-patients with in-stent stenosis were retreated with balloon angioplasty. Stenosis grade and postprocedural antiplatelet therapy of the reported in-stent stenosis in the CWS-group are summarized in Table 3.
Discussion
This investigation demonstrates that the deployment of CGuard stent was safe in the setting of emergent CAS. Compared to other reported dual-layer stents, the rate of acute in-stent-occlusions was not increased, but on the contrary lower when compared to the mono-layer CWS, however without statistical significance (4.8%vs. 9.1%). The rate of in-stent-occlusions after acute CAS with CGuard stent was lower in our series than in the single-center series including 33 patients ( 9% acute occlusion rate) reported by Klail et al. [22]. The rate of in-stent occlusions of CWS in our series was in range with the findings of previous studies [23]. However, this current acute in-stent-occlusion rate with the CGuard appears to be in direct opposition with the high occlusion rates with other DLSs (Casper-RX, Microvention) reported by Bartolini et al. (52.4%) [16]. Furthermore, Pfaff et al. reported in a multicentric study thrombus formation and complete occlusion of the Casper-RX during the procedure or within 72 h in 33/160 (20.8%) and in 12/160 patients (7.5%), respectively [15]. These alarming results lead to call from some authors for the discontinuation of DLSs [16].
For the purpose of analyzing the varying occlusion rates among different DLSs, it is important to understand the crucial differences in stent design. The Casper-RX stent is formed from a braided metal frame with nickel-titanium alloy mesh layer within the stent frame, whereas the protective, single-fiber knitted mesh in the CGuard-stent encompasses the frame of the stent from the outside. That means that CGuard stent has considerably less thrombogenic material and its outer mesh layer offers structural support. This can explain the previously reported higher rates of in-stent restenosis reported in other DLSs [24, 25]. The hybrid stent design in CGuard, meaning that the open cell component (free area 16.25 mm2 vs. 1.09 mm2 of CWS) offers flexibility and conformability especially in torturous vessels, something that has been limited for instance in closed-cell stents [26].
The rate of in-stent stenosis on the follow-up imaging at 6 months was higher in the CWS-group compared to the CGuard group (6 vs.0) and statistically significant (p = 0.039). One possible explanation is that the better coverage of the DLS prevents protrusion of the fresh thrombotic material more efficiently. However, due to the different antithrombotic regimes in this investigation and the low number of included follow-ups we are reluctant to overinterpret these results.
In the current investigation, post-dilation was considered when a restenosis > 50% occurred after deployment before the end of the intervention. There are several benefits and considerable risks related to post-dilation angioplasties. On one hand, it is thought to reduce the incidence of restenosis by re-establishing the normal diameter of the vessel through closely apposing the stent and intima [27]. However, several investigations have found that post-dilation increases the risk of silent ischemia and neurological events [28], as it presents a high risk for embolization when the balloon pushes the stent struts against the atheromatous plaque [29].
In this investigation, the underlying pathology for the acute occlusion or high-grade stenosis was predominantly atherosclerosis n = 81 (94.2%), in addition to 5 cases (5.8%) of acute dissection of the cervical ICA. Although, both represent very different pathologies. However, in clinical routine and in particular in an emergency setting it is often not easy to distinguish between both. Gory et al. did not find a significant difference between dissection and atherosclerotic tandem occlusions in the multi-centric evaluation of 295 patients (65 patients with a dissection) concerning clinical outcome, hemorrhage and mortality; however, there is no information on early re-occlusion in this cohort [30]. Similarly, it was found in the TITAN registry that the etiology of the cervical ICA lesion (atherosclerosis vs. dissection) did not impact the final reperfusion rates or clinical outcomes [31].
There was a significant difference between both groups with regards to number of accompanying intracranial ICA occlusions CWS 29.5% (n = 13) vs. 11.9% (n = 5) of CGuard. In total, CWS group included 4 cases of ICA-L and 9 of ICA-T occlusions, whereas all the intracranial ICA-occlusions in the CGuard group were ICA-T occlusions. ICA-T occlusions have been associated with a poor outcome as shown in previous observational thrombectomy studies [32, 33]. This may contribute to the fact that CWS patients had a worse clinical outcome post-procedurally.
There has been sufficient evidence that in-stent occlusions especially among patients with tandem occlusions after acute CAS stenting is significantly less frequent among patients receiving DAPT when compared to patients receiving mono antiplatelet therapy (MAPT) [34]. Neurointerventionalists face a conundrum. On one hand, dual antiplatelet therapy has been widely used to prevent in-stent thrombosis after aCAS [35]. On the other hand, pretreatment antiplatelet therapy before EVT is associated with an increased risk of ICH [36].
In this study, both stent groups received ASA during stent deployment followed by DAPT after the follow-up CT as a standard therapy for the prevention of in-stent occlusion [37]. The CWS group received predominantly ASA and clopidogrel, whereas the CGuard group received ASA and Ticagrelor. None of the DAPT variants were associated with an increased risk of in-stent occlusions excluding potential bias from this heterogeneity.
In our series, 3 out of 6 patients with an in-stent-thrombosis had an underlying dissection thus indicating that stent-implantation under monotherapy (no matter if it is the wall-stent or the CGuard) might be unsafe and should be potentially performed under a more aggressive antiplatelet regime, e.g. GPIIb/IIIa inhibitors or dual antiplatelets. However, this more aggressive medication may translate in an increased bleeding rate and must – at least before we have more reliable data - be carefully weighted in the individual case.
Furtherly, we did not find a higher rate of in-stent occlusion in patients who did not receive IV tPA in the current investigation, which is different from the findings reported by Yilmaz et al. [14].
Thus, we could not identify which DAPT is more thrombo-protective. This is relevant, because while Ticagrelor is potent, has a quicker onset than clopidogrel [38] and overcomes the issue of non-or poor-responders [39], its cost is significantly higher.
It is unfortunate in this investigation, that only 15/42 (35.7%) of the CGuard group and 18/44 (40.9%) of the CWS-group received follow-up imaging at 6 months. This is attributed to the high quota of external stroke patients referred to the current comprehensive stroke center for treatment, which are consecutively transferred back to their local hospitals and neurological rehabilitation centers after treatment.
Limitations
There are several limitations: first, the collected data were from a single-center and analyzed retrospectively. The small number of observed occlusions in both patient groups introduce a severe data fragility. The retrospective nature of the study implies a number of limitations in the form of different patient characteristics, reported and unreported interventional technical aspects of the underlying procedures and heterogenous peri- and postinterventional medication regimes, as it was decided at the discretion of the treating neurologist and neuroradiologist, possibly influencing patient outcomes as well as the endpoints in-stent occlusion rate and intracranial hemorrhage rate. Second, missing further follow-up data prevent the assessment of stent patency mid- and long-term. Third, the modalities used to evaluate stent patency have varying sensitivity.
Conclusions
We present the so far largest cohort of patients who were treated with the dual-layer CGuard-stent in an emergent setting, mainly in tandem occlusions. We were able to demonstrate that the in-stent occlusion rate was lower with the CGuard stent than reported with other dual-layer stents and comparable to the single-layered CWS. Further data are needed to evaluate the potential benefit of the design in more detail.
Data availability
Individual participant data that underlie the results reported in this article (after deidentification) will be available upon reasonable request. These proposals should be directed to the corresponding author.
References
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Davalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millan M, Davis SM, Roy D, Thornton J, Roman LS, Ribo M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG (2016) collaborators H Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387(10029):1723-31. https://doi.org/10.1016/S0140-6736(16)00163-X
Hebert D, Elder TA, Adel JG (2022) Emergent carotid endarterectomy and mechanical thrombectomy in tandem occlusion. Surg Neurol Int 13:521. https://doi.org/10.25259/SNI_740_2022
Jadhav AP, Zaidat OO, Liebeskind DS, Yavagal DR, Haussen DC, Hellinger FR Jr., Jahan R, Jumaa MA, Szeder V, Nogueira RG, Jovin TG (2019) Emergent management of tandem lesions in acute ischemic stroke. Stroke 50(2):428–433. https://doi.org/10.1161/STROKEAHA.118.021893
Heck DV, Brown MD (2015) Carotid stenting and intracranial thrombectomy for treatment of acute stroke due to tandem occlusions with aggressive antiplatelet therapy may be associated with a high incidence of intracranial hemorrhage. J Neurointerv Surg 7(3):170–175. https://doi.org/10.1136/neurintsurg-2014-011224
Lockau H, Liebig T, Henning T, Neuschmelting V, Stetefeld H, Kabbasch C, Dorn F (2015) Mechanical thrombectomy in tandem occlusion: procedural considerations and clinical results. Neuroradiology 57(6):589–598. https://doi.org/10.1007/s00234-014-1465-5
Collette SL, Rodgers MP, van Walderveen MAA, Compagne KCJ, Nederkoorn PJ, Hofmeijer J, Martens JM, de Borst GJ, Luijckx GJR, Majoie C, van der Lugt A, Bokkers RPH, Uyttenboogaart M, investigators MCR (2023) Management of extracranial carotid artery stenosis during endovascular treatment for acute ischaemic stroke: results from the MR CLEAN Registry. Stroke Vasc Neurol 8(3):229–237. https://doi.org/10.1136/svn-2022-001891
Papanagiotou P, Haussen DC, Turjman F, Labreuche J, Piotin M, Kastrup A, Steglich-Arnholm H, Holtmannspotter M, Taschner C, Eiden S, Nogueira RG, Boutchakova M, Siddiqui A, Lapergue B, Dorn F, Cognard C, Killer M, Mangiafico S, Ribo M, Psychogios MN, Spiotta A, Labeyrie MA, Biondi A, Mazighi M, Richard S, Anxionnat R, Bracard S, Gory B, Investigators T (2018) Carotid stenting with antithrombotic agents and intracranial thrombectomy leads to the highest recanalization rate in patients with acute stroke with tandem lesions. JACC Cardiovasc Interv 11(13):1290–1299. https://doi.org/10.1016/j.jcin.2018.05.036
Quispe-Orozco D, Limaye K, Zevallos CB, Farooqui M, Mendez-Ruiz A, Ansari S, Dajles A, Samaniego EA, Derdeyn C, Ortega-Gutierrez S (2021) Safety and efficacy of symptomatic carotid artery stenting performed in an emergency setting. Interv Neuroradiol 27(3):411–418. https://doi.org/10.1177/1591019920977552
Feil K, Herzberg M, Dorn F, Tiedt S, Kupper C, Thunstedt DC, Papanagiotou P, Meyer L, Kastrup A, Dimitriadis K, Liebig T, Dieterich M, Kellert L, investigatorsdagger GSR (2021) Tandem lesions in anterior circulation stroke: analysis of the German stroke registry-endovascular treatment. Stroke 52(4):1265–1275. https://doi.org/10.1161/STROKEAHA.120.031797
Anadani M, Marnat G, Consoli A, Papanagiotou P, Nogueira RG, Siddiqui A, Ribo M, Spiotta AM, Bourcier R, Kyheng M, Labreuche J, de Havenon A, Sibon I, Dargazanli C, Arquizan C, Cognard C, Olivot JM, Anxionnat R, Audibert G, Mazighi M, Blanc R, Lapergue B, Richard S, Gory B, Titan, Investigators ER (2021) Endovascular therapy of anterior circulation tandem occlusions: pooled analysis from the TITAN and ETIS registries. Stroke 52(10):3097–3105. https://doi.org/10.1161/STROKEAHA.120.033032
Wallocha M, Chapot R, Nordmeyer H, Fiehler J, Weber R, Stracke CP (2019) Treatment methods and early neurologic improvement after endovascular treatment of tandem occlusions in acute ischemic stroke. Front Neurol 10:127. https://doi.org/10.3389/fneur.2019.00127
Poppe AY, Jacquin G, Roy D, Stapf C, Derex L (2020) Tandem carotid lesions in acute ischemic stroke: mechanisms, therapeutic challenges, and future directions. AJNR Am J Neuroradiol 41(7):1142–1148. https://doi.org/10.3174/ajnr.A6582
Wodarg F, Turner EL, Dobson J, Ringleb PA, Mali WP, Fraedrich G, Chatellier G, Bequemin JP, Brown MM, Algra A, Mas JL, Jansen O, Bonati LH, Carotid Stenosis Trialists C (2018) Influence of stent design and use of protection devices on outcome of carotid artery stenting: a pooled analysis of individual patient data. J Neurointerv Surg 10(12):1149–1154. https://doi.org/10.1136/neurintsurg-2017-013622
Yilmaz U, Korner H, Muhl-Benninghaus R, Simgen A, Kraus C, Walter S, Behnke S, Fassbender K, Reith W, Unger MM (2017) Acute occlusions of dual-layer carotid stents after endovascular emergency treatment of tandem lesions. Stroke 48(8):2171–2175. https://doi.org/10.1161/STROKEAHA.116.015965
Pfaff JAR, Maurer C, Broussalis E, Janssen H, Blanc R, Dargazanli C, Costalat V, Piotin M, Runck F, Berlis A, Killer-Oberpfalzer M, Hensler JT, Bendszus M, Wodarg F, Mohlenbruch MA (2020) Acute thromboses and occlusions of dual layer carotid stents in endovascular treatment of tandem occlusions. J Neurointerv Surg 12(1):33–37. https://doi.org/10.1136/neurintsurg-2019-015032
Bartolini B, Puccinelli F, Mosimann PJ, Hajdu SD, Veunac L, Michel P, Saliou G (2019) Evaluating the effectiveness and safety of the carotid casper-RX stent for tandem lesions in acute ischemic stroke. J Neurointerv Surg 11(8):772–774. https://doi.org/10.1136/neurintsurg-2018-014425
Schofer J, Musialek P, Bijuklic K, Kolvenbach R, Trystula M, Siudak Z, Sievert H (2015) A prospective, multicenter study of a novel mesh-covered carotid stent: the CGuard CARENET trial (Carotid embolic protection using MicroNet). JACC Cardiovasc Interv 8(9):1229–1234. https://doi.org/10.1016/j.jcin.2015.04.016
Sirignano P, Stabile E, Mansour W, Capoccia L, Faccenna F, Intrieri F, Ferri M, Sacca S, Sponza M, Mortola P, Ronchey S, Praquin B, Grillo P, Chiappa R, Losa S, Setacci F, Pirrelli S, Taurino M, Ruffino MA, Udini M, Palombo D, Ippoliti A, Montelione N, Setacci C, de Donato G, Ruggeri M, Speziale F (2021) 1-year results from a prospective experience on CAS using the CGuard stent system: the IRONGUARD 2 study. JACC Cardiovasc Interv 14(17):1917–1923. https://doi.org/10.1016/j.jcin.2021.05.045
Klail T, Kurmann C, Kaesmacher J, Mujanovic A, Piechowiak EI, Dobrocky T, Pilgram-Pastor S, Scutelnic A, Heldner MR, Gralla J, Mordasini P (2023) Safety and efficacy of carotid artery stenting with the CGuard double-layer stent in acute ischemic stroke. Clin Neuroradiol 33(1):237–244. https://doi.org/10.1007/s00062-022-01209-3
Montorsi P, Caputi L, Galli S, Ravagnani PM, Teruzzi G, Annoni A, Calligaris G, Fabbiocchi F, Trabattoni D, de Martini S, Grancini L, Pontone G, Andreini D, Troiano S, Restelli D, Bartorelli AL (2020) Carotid wallstent versus roadsaver stent and distal versus proximal protection on cerebral microembolization during carotid artery stenting. JACC Cardiovasc Interv 13(4):403–414. https://doi.org/10.1016/j.jcin.2019.09.007
Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D, Investigators E (2008) Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 359(13):1317–1329. https://doi.org/10.1056/NEJMoa0804656
Klail T, Kurmann C, Kaesmacher J, Mujanovic A, Piechowiak EI, Dobrocky T, Pilgram-Pastor S, Scutelnic A, Heldner MR, Gralla J, Mordasini P (2022) Safety and efficacy of carotid artery stenting with the CGuard double-layer stent in acute ischemic stroke. Clin Neuroradiol 1–8. https://doi.org/10.1007/s00062-022-01209-3
Steglich-Arnholm H, Holtmannspotter M, Kondziella D, Wagner A, Stavngaard T, Cronqvist ME, Hansen K, Hojgaard J, Taudorf S, Krieger DW (2015) Thrombectomy assisted by carotid stenting in acute ischemic stroke management: benefits and harms. J Neurol 262(12):2668–2675. https://doi.org/10.1007/s00415-015-7895-0
Sýkora J, Zeleňák K, Vorčák M, Števík M, Sýkorová M, Sivák J, Rovňák M, Zapletalová J, Mužík J, Šinák I, Kurča E, Meyer L, Fiehler J (2022) Comparison of restenosis risk in single-layer versus dual-layer carotid stents: a duplex ultrasound evaluation. Cardiovasc Intervent Radiol 45(9):1257–1266. https://doi.org/10.1007/s00270-022-03200-4
Stabile E, de Donato G, Musialek P, Deloose K, Nerla R, Sirignano P, Mazurek A, Mansour W, Fioretti V, Esposito F, Chianese S, Bosiers M, Setacci C, Speziale F, Micari A, Esposito G (2020) Use of dual-layered stents for carotid artery angioplasty: 1-year results of a patient-based meta-analysis. JACC Cardiovasc Interv 13(14):1709–1715. https://doi.org/10.1016/j.jcin.2020.03.048
Faateh M, Dakour-Aridi H, Mathlouthi A, Locham S, Naazie I, Malas M (2021) Comparison of open- and closed-cell stent design outcomes after carotid artery stenting in the vascular quality initiative. J Vasc Surg 73(5):1639–1648. https://doi.org/10.1016/j.jvs.2020.08.155
Phatouros CC, Higashida RT, Malek AM, Meyers PM, Lempert TE, Dowd CF, Halbach VV (2000) Carotid artery stent placement for atherosclerotic disease: rationale, technique, and current status. Radiology 217(1):26–41. https://doi.org/10.1148/radiology.217.1.r00oc2526
Besli F, Gungoren F, Kocaturk O, Tanriverdi Z, Tascanov MB (2019) The impact of post-dilatation on periprocedural outcomes during carotid artery stenting: a single-center experience. Atherosclerosis 290:74–79. https://doi.org/10.1016/j.atherosclerosis.2019.09.024
Ackerstaff RG, Suttorp MJ, van den Berg JC, Overtoom TT, Vos JA, Bal ET, Zanen P, Antonius Carotid Endarterectomy A, Stenting Study G (2005) Prediction of early cerebral outcome by transcranial doppler monitoring in carotid bifurcation angioplasty and stenting. J Vasc Surg 41(4):618–624. https://doi.org/10.1016/j.jvs.2005.01.034
Gory B, Piotin M, Haussen DC, Steglich-Arnholm H, Holtmannspotter M, Labreuche J, Taschner C, Eiden S, Nogueira RG, Papanagiotou P, Boutchakova M, Siddiqui A, Lapergue B, Dorn F, Cognard C, Killer-Oberpfalzer M, Mangiafico S, Ribo M, Behme D, Spiotta AM, Mazighi M, Turjman F, Investigators T (2017) Thrombectomy in acute stroke with tandem occlusions from dissection versus atherosclerotic cause. Stroke 48(11):3145–3148. https://doi.org/10.1161/STROKEAHA.117.018264
Zhu F, Bracard S, Anxionnat R, Derelle AL, Tonnelet R, Liao L, Mione G, Humbertjean L, Lacour JC, Hossu G, Anadani M, Richard S, Gory B (2019) Impact of emergent cervical carotid stenting in tandem occlusion strokes treated by thrombectomy: a review of the TITAN collaboration. Front Neurol 10:206. https://doi.org/10.3389/fneur.2019.00206
Vavrova J, Koznar B, Peisker T, Vasko P, Rohac F, Sulzenko J, Kroupa J, Stetkarova I, Mengozzi L, Petras M, Widimsky P (2021) Long-term outcomes of thrombectomy for acute ischaemic stroke by occluded artery and stroke aetiology: a PRAGUE-16 substudy. EuroIntervention 17(2):e169–e77. https://doi.org/10.4244/EIJ-D-19-00997
Fischer U, Mono ML, Schroth G, Jung S, Mordasini P, El-Koussy M, Weck A, Brekenfeld C, Findling O, Galimanis A, Heldner MR, Arnold M, Mattle HP, Gralla J (2013) Endovascular therapy in 201 patients with acute symptomatic occlusion of the internal carotid artery. Eur J Neurol 20(7):1017–1024. https://doi.org/10.1111/ene.12094
Pop R, Zinchenko I, Quenardelle V, Mihoc D, Manisor M, Richter JS, Severac F, Simu M, Chibbaro S, Rouyer O, Wolff V, Beaujeux R (2019) Predictors and clinical impact of delayed stent thrombosis after thrombectomy for acute stroke with tandem lesions. AJNR Am J Neuroradiol 40(3):533–539. https://doi.org/10.3174/ajnr.A5976
Goyal M, Yoshimura S, Milot G, Fiehler J, Jayaraman M, Dorn F, Taylor A, Liu J, Albuquerque F, Jensen ME, Nogueira R, Fraser JF, Chapot R, Thibault L, Majoie C, Yang P, Sakai N, Kallmes D, Orlov K, Arthur A, Brouwer P, Ospel JM (2020) Considerations for antiplatelet management of carotid stenting in the setting of mechanical thrombectomy: a Delphi consensus statement. AJNR Am J Neuroradiol 41(12):2274–2279. https://doi.org/10.3174/ajnr.A6888
Mulder MJ, Berkhemer OA, Fransen PS, van den Berg LA, Lingsma HF, den Hertog HM, Staals J, Jenniskens SF, van Oostenbrugge RJ, van Zwam WH, Majoie CB, van der Lugt A, Dippel DW, investigators MC (2017) Does prior antiplatelet treatment improve functional outcome after intra-arterial treatment for acute ischemic stroke? Int J Stroke 12(4):368–376. https://doi.org/10.1177/1747493016677842
Jeger RV, Pfisterer ME, Sorensen R, von Felten S, Alber H, Bonetti PO, Eberli F, Erne P, Pedrazzini G, Rickli H, Galatius S, Kaiser CA, Basket, investigators B-P (2014) Tradeoff between bleeding and stent thrombosis in different dual antiplatelet therapy regimes: importance of case fatality rates and effective treatment durations. Am Heart J 168(5):698–705. https://doi.org/10.1016/j.ahj.2014.07.019
Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Investigators P, Freij A, Thorsen M (2009) Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 361(11):1045–1057. https://doi.org/10.1056/NEJMoa0904327
Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, Husted S, Katus H, Steg PG, Shah SH, Becker RC (2010) investigators P Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet 376(9749):1320-8. https://doi.org/10.1016/S0140-6736(10)61274-3
Mousa Z, Paech D, Bode F, Gronemann C, Petzold G, Dorn F (2023) P166/253 safety and efficacy of the dual-layer CGuard stent for the treatment of tandem occlusions – a comparative study with the carotid wallstent. J Neurointerv Surg 15(Suppl 2):A78–A9. https://doi.org/10.1136/jnis-2023-ESMINT.194
Acknowledgements
There are no acknowledgments.
Funding
No funding.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Mousa Zidan: Methodology, Data curation, Formal Analysis, Writing- Original draft preparation. Christian Gronemann: Data acquisition. Felix Bode, Johannes Weller: Conceptualization, Validation. Nils Lehnen: Investigation, Validation. Gabor Petzold, Alexander Radbruch: Supervision, Franziska Dorn, Daniel Paech: Methodology, Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Ethics approval and informed consent
Ethical approval for this retrospective study was waived by the Ethics Committee of Bonn University. Research was conducted according to the principles of the declaration of Helsinki. Due to the retrospective character of the data acquisition and analysis and emphasis on patient safety and quality control as well, written informed consent was waived.
Conflict of interest
FD serves as a proctor/consultant for Cerenovus, Microvention, Balt, Cerus Endovascular, received scientific grant from Cerenovus and speaker’s honoraria from Acandis, Asahi, Stryker. The authors stated explicitly that there are no conflicts of interest in connection with this article.
The included abstract has been presented at the European Society of Minimally Invasive Neurological Therapy (ESMINT) 2023 and published in its corresponding journal [40].
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zidan, M., Gronemann, C., Lehnen, N. et al. Stenting with dual-layer CGuard stent in acute sub-occlusive carotid artery stenosis and in tandem occlusions: a monocentric study. Neuroradiology (2024). https://doi.org/10.1007/s00234-024-03397-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00234-024-03397-w