Abstract
Purpose
In brain development, Myelination is the characteristic feature of white matter maturation, which plays an important role in efficient information transmitting. The white matter abnormality has been reported to be associated with self-limited epilepsy with centrotemporal spikes (SeLECTS). This study aimed to detect the altered white matter region in the SeLECTS patients by the combination of diffusion tensor imaging (DTI) and quantitative susceptibility mapping (QSM) technique.
Methods
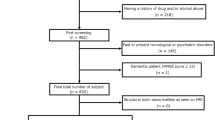
27 children with SeLECTS and 23 age- and gender-matched healthy children were enrolled. All participants were scanned with 3.0-T MRI to acquire the structure, diffusion and susceptibility-weighted data. The susceptibility and diffusion weighted data were processed to obtain quantitative susceptibility map and fraction anisotropy (FA) map. Then voxel-wise tract-based spatial statistics (TBSS) were used to analyze quantitative susceptibility and FA data.
Results
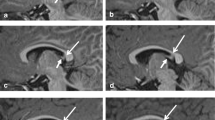
Both DTI and QSM revealed extensive white matter alterations in the frontal, parietal, and temporal lobes in SeLECTS patients. The overlapped region of DTI and QSM analyses was located in the fiber tracts of the corona radiata. The FA values in this overlapped region were negatively correlated with the magnetic susceptibility values.
Conclusion
Our results suggest that TBSS-based QSM can be employed as a novel approach for characterizing alterations in white matter in SeLECTS. And the combination of QSM and DTI can provide a more comprehensive evaluation of white matter integrity by utilizing different biophysical features.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- SeLECTS:
-
Self-limited epilepsy with centrotemporal spikes
- DTI:
-
Diffusion Tensor Imaging
- FA:
-
Fractional Anisotropy
- QSM:
-
Quantitative Susceptibility Mapping
- HC:
-
Healthy Controls
- MNI:
-
Montreal Neurological Institute
- FSL:
-
FMRIB Software Library
- TBSS:
-
Tract Based Spatial Statistics
- ASMs:
-
Antiseizure medications
References
Specchio N, Wirrell EC, Scheffer IE et al (2022) International league against epilepsy classification and definition of epilepsy syndromes with onset in childhood: position paper by the ilae task force on nosology and definitions. Epilepsia 63:1398–1442
Dalla Bernardina B, Sgrò V, Caraballo R et al (1991) Sleep and benign partial epilepsies of childhood: EEG and evoked potentials study. Epilepsy Res Suppl 2:83–96
Wirrell EC, Grossardt BR, Wong Kisiel LCL, Nickels KC (2011) Incidence and classification of new-onset epilepsy and epilepsy syndromes in children in Olmsted County, Minnesota from 1980 to 2004:a population-based study. Epilepsy Res 95:110–118
Kavros PM, Clarke T, Strug LJ, Halperin JM, Dorta NJ, Pal DK (2008) Attention impairment in rolandic epilepsy: systematic review. Epilepsia 49:1570–1580
Yung AW, Park YD, Cohen MJ, Garrison TN (2000) Cognitive and behavioral problems in children with centrotemporal spikes. Pediatr Neurol 23:391–395
Kim SE, Lee JH, Chung HK et al (2014) Alterations in white matter microstructures and cognitive dysfunctions in benign childhood epilepsy with centrotemporal spikes. Eur J Neurol 21:708–717
Zhang Q, He Y, Qu T et al (2021) Delayed brain development of Rolandic epilepsy profiled by deep learning–based neuroanatomic imaging. Eur Radiol 31:9628–9637
Pardoe HR, Berg AT, Archer JS, Fulbright RK, Jackson GD (2013) A neurodevelopmental basis for BECTS: evidence from structural MRI. Epilepsy Res 105:133–139
Wilent WB, Contreras D (2005) Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nat Neurosci 8:1364–1370
Lenroot RK, Giedd JN (2006) Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav R 30:718–729
Gogtay N, Giedd JN, Lusk L et al (2004) Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci 101:8174–8179
Ciumas C, Saignavongs M, Ilski F et al (2014) White matter development in children with benign childhood epilepsy with centro-temporal spikes. Brain 137:1095–1106
Eluvathingal TJ, Hasan KM, Kramer L et al (2007) Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 17:2760–2768
Qiu A, Mori S, Miller MI (2015) Diffusion tensor imaging for understanding brain development in early life. Annu Rev Psychol 66:853–876
Xiao F, Chen Q, Yu X et al (2014) Hemispheric lateralization of microstructural white matter abnormalities in children with active benign childhood epilepsy with centrotemporal spikes (BECTS): a preliminary DTI study. J Neurol sci 336:171–179
Yu FF, Chiang FL, Stephens N et al (2019) Characterization of normal-appearing white matter in multiple sclerosis using quantitative susceptibility mapping in conjunction with diffusion tensor imaging. Neuroradiology 61:71–79
Yoshida S, Oishi K, Faria AV, Mori S (2013) Diffusion tensor imaging of normal brain development. Pediatr Radiol 43:15–27
Rudko DA, Solovey I, Gati JS, Kremenchutzky M, Menon RS (2014) Multiple sclerosis: improved identification of diseaserelevant changes in gray and white matter by using susceptibilitybased MR imaging. Radiology 272:851–864
Argyridis I, Li W, Johnson GA, Liu C (2013) Quantitative magnetic susceptibility of the developing mouse brain reveals microstructural changes in the white matter. Neuroimage 88:134–142
Li W, Avram AV, Wu B, Xiao X, Liu C (2014) Integrated Laplacianbased phase unwrapping and background phase removal for quantitative susceptibility mapping. NMR Biomed 27:219–227
Li W, Wu B, Avram AV, Liu C (2012) Magnetic susceptibility anisotropy of human brain in vivo and its molecular underpinnings. Neuroimage 59:2088–2097
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008) Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40:1044–1055
Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA (2012) Development of white matter and reading skills. Proc Natl Acad Sci 109:E3045-3053
Lebel C, Gee M, Camicioli R et al (2012) Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage 60:340–352
Govindan RM, Makki MI, Sundaram SK, Juhász C, Chugani HT (2008) Diffusion tensor analysis of temporal and extra-temporal lobe tracts in temporal lobe epilepsy. Epilepsy Res 80:30–41
Shu M, Yu C, Shi Q, Li Y, Niu K, Zhang S, Wang X (2021) Alterations in white matter integrity and asymmetry in patients with benign childhood epilepsy with centrotemporal spikes and childhood absence epilepsy: An automated fiber quantification tractography study. Epilepsy Behav 123:108235
Thorn EL, Ostrowski LM, Chinappen DM et al (2020) Persistent abnormalities in Rolandic thalamocortical white matter circuits in childhood epilepsy with centrotemporal spikes. Epilepsia 61:2500–2508
Agarwal R, Kumar A, Tiwari VN, Chugani H (2016) Thalamic abnormalities in children with continuous spike-wave during slow-wave sleep: an F-18-fluorodeoxyglucose positron emission tomography perspective. Epilepsia 57:263–271
Funding
This study has received funding by Guizhou Provincial Science and Technology Projects (grant number [2020] 1Y346).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Ethical approval
This study was approved by the institutional review board of the affiliated hospital of zunyi medical university (No. 1-047).
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, G., Zhang, Y. & Chen, X. Combined diffusion tensor imaging and quantitative susceptibility mapping to characterize normal-appearing white matter in self-limited epilepsy with centrotemporal spikes. Neuroradiology (2024). https://doi.org/10.1007/s00234-024-03367-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00234-024-03367-2