Abstract
Introduction
We aimed to investigate the alterations in the multilayer network in patients with transient global amnesia (TGA).
Methods
We enrolled 124 patients with TGA and 80 healthy controls. Both patients with TGA and healthy controls underwent a three-teslar brain magnetic resonance imaging (MRI). A gray matter layer matrix was created using a morphometric similarity network derived from the T1-weighted imaging, and a white matter layer matrix was constructed using structural connectivity based on the diffusion tensor imaging. A multilayer network analysis was performed by applying graph theoretical analysis.
Results
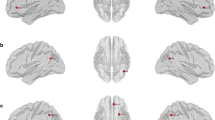
There were no significant differences in global network measures between the groups. However, several regions, related to the default mode network, showed significant differences in nodal network measures between the groups. Multi-richness in the left pars opercularis, multi-rich-club degree in the right posterior cingulate gyrus, and weighted multiplex participation in the right posterior cingulate gyrus were higher in patients with TGA compared with healthy controls (15.47 vs. 12.26, p = 0.0005; 41.68 vs. 37.16, p = 0.0005; 0.90 vs. 0.80, p = 0.0005; respectively). The multiplex core–periphery in the left precuneus was higher (0.96 vs. 0.84, p = 0.0005), whereas that in the transverse temporal gyrus was lower in patients with TGA compared with healthy controls (0.00 vs. 0.02, p = 0.0005).
Conclusion
We newly find the alterations in the multilayer network in patients with TGA compared with healthy controls, which shows the involvement of the default mode network. These changes may be related to the pathophysiology of TGA.


Similar content being viewed by others
Data availability
Data that support the findings of this study are available upon reasonable request.
References
Kirshner HS (2011) Transient global amnesia: a brief review and update. Curr Neurol Neurosci Rep 11(6):578–582. https://doi.org/10.1007/s11910-011-0224-9
Huang CF, Pai MC (2008) Transient amnesia in a patient with left temporal tumor: symptomatic transient global amnesia or an epileptic amnesia? Neurologist 14(3):196–200. https://doi.org/10.1097/NRL.0b013e3181618af1
Chau L, Liu A (2019) Transient global amnesia as the sole presentation of an acute stroke in the left cingulate gyrus. Case Rep Neurol Med 2019:4810629. https://doi.org/10.1155/2019/4810629
Meng D, Alsaeed M, Randhawa J, Chen T (2021) Retrosplenial stroke mimicking transient global amnesia. Can J Neurol Sci 48(6):884–885. https://doi.org/10.1017/cjn.2021.6
Teive HA, Kowacs PA, Maranhao Filho P, Piovesan EJ, Werneck LC (2005) Leao’s cortical spreading depression: from experimental “artifact” to physiological principle. Neurology 65(9):1455–1459. https://doi.org/10.1212/01.wnl.0000183281.12779.cd
Lewis SL (1998) Aetiology of transient global amnesia. Lancet 352(9125):397–399. https://doi.org/10.1016/S0140-6736(98)01442-1
Pantoni L, Bertini E, Lamassa M, Pracucci G, Inzitari D (2005) Clinical features, risk factors, and prognosis in transient global amnesia: a follow-up study. Eur J Neurol 12(5):350–356. https://doi.org/10.1111/j.1468-1331.2004.00982.x
Seo YD, Lee DA, Park KM (2023) Can artificial intelligence diagnose transient global amnesia using electroencephalography data? J Clin Neurol 19(1):36–43. https://doi.org/10.3988/jcn.2023.19.1.36
Park KM, Han YH, Kim TH, Mun CW, Shin KJ, Ha SY, Park J, Kim SE (2015) Pre-existing structural abnormalities of the limbic system in transient global amnesia. J Clin Neurosci 22(5):843–847. https://doi.org/10.1016/j.jocn.2014.11.017
Kim HC, Lee BI, Kim SE, Park KM (2017) Cortical morphology in patients with transient global amnesia. Brain Behav 7(12):e00872. https://doi.org/10.1002/brb3.872
Park KM, Lee BI, Kim SE (2018) Is transient global amnesia a network disease? Eur Neurol 80(5–6):345–354. https://doi.org/10.1159/000496511
Kim J, Lee DA, Kim HC, Lee HJ, Park KM (2021) Brain networks in patients with isolated or recurrent transient global amnesia. Acta Neurol Scand 144(5):465–472. https://doi.org/10.1111/ane.13490
Kim GH, Kim BR, Chun MY, Park KD, Lim SM, Jeong JH (2021) Aberrantly higher functional connectivity in the salience network is associated with transient global amnesia. Sci Rep 11(1):20598. https://doi.org/10.1038/s41598-021-97842-y
Logan AP, LaCasse PM, Lunday BJ (2023) Social network analysis of Twitter interactions: a directed multilayer network approach. Soc Netw Anal Min 13(1):65. https://doi.org/10.1007/s13278-023-01063-2
Buldu JM, Papo D (2018) Can multilayer brain networks be a real step forward?: Comment on “Network science of biological systems at different scales: A review” by M. Gosak et al. Phys Life Rev 24:153–155
Klepl D, He F, Wu M, Blackburn DJ, Sarrigiannis PG (2023) Cross-frequency multilayer network analysis with bispectrum-based functional connectivity: a study of Alzheimer’s disease. Neuroscience 521:77–88. https://doi.org/10.1016/j.neuroscience.2023.04.008
Long D, Zhang M, Yu J, Zhu Q, Chen F, Li F (2023) Intelligent diagnosis of major depression disease based on multi-layer brain network. Front Neurosci 17:1126865. https://doi.org/10.3389/fnins.2023.1126865
Ke M, Wang C, Liu G (2023) Multilayer brain network modeling and dynamic analysis of juvenile myoclonic epilepsy. Front Behav Neurosci 17:1123534. https://doi.org/10.3389/fnbeh.2023.1123534
Hodges JR, Warlow CP (1990) Syndromes of transient amnesia: towards a classification A study of 153 cases. J Neurol Neurosurg Psychiatry 53(10):834–843. https://doi.org/10.1136/jnnp.53.10.834
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97(20):11050–11055. https://doi.org/10.1073/pnas.200033797
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31(3):968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021
King DJ, Wood AG (2020) Clinically feasible brain morphometric similarity network construction approaches with restricted magnetic resonance imaging acquisitions. Netw Neurosci 4(1):274–291. https://doi.org/10.1162/netn_a_00123
Seidlitz J, Vasa F, Shinn M, Romero-Garcia R, Whitaker KJ, Vertes PE, Wagstyl K, Kirkpatrick Reardon P, Clasen L, Liu S, Messinger A, Leopold DA, Fonagy P, Dolan RJ, Jones PB, Goodyer IM, Consortium N, Raznahan A, Bullmore ET (2018) Morphometric similarity networks detect microscale cortical organization and predict inter-individual cognitive variation. Neuron 97(1):231-247e237. https://doi.org/10.1016/j.neuron.2017.11.039
Yeh FC, Wedeen VJ, Tseng WY (2010) Generalized q-sampling imaging. IEEE Trans Med Imaging 29(9):1626–1635. https://doi.org/10.1109/TMI.2010.2045126
Mijalkov M, Kakaei E, Pereira JB, Westman E, Volpe G (2017) Alzheimer’s Disease Neuroimaging I BRAPH: a graph theory software for the analysis of brain connectivity. PLoS One 12(8):0178798. https://doi.org/10.1371/journal.pone.0178798
de Domenico M (2018) Multilayer network modeling of integrated biological systems: Comment on “Network science of biological systems at different scales: A review” by Gosak et al. Phys Life Rev 24:149–152
Puxeddu MG, Petti M, Astolfi L (2021) A comprehensive analysis of multilayer community detection algorithms for application to EEG-based brain networks. Front Syst Neurosci 15:624183. https://doi.org/10.3389/fnsys.2021.624183
Lv Y, Huang S, Zhang T, Gao B (2021) Application of multilayer network models in bioinformatics. Front Genet 12:664860. https://doi.org/10.3389/fgene.2021.664860
Casas-Roma J, Martinez-Heras E, Sole-Ribalta A, Solana E, Lopez-Soley E, Vivo F, Diaz-Hurtado M, Alba-Arbalat S, Sepulveda M, Blanco Y, Saiz A, Borge-Holthoefer J, Llufriu S, Prados F (2022) Applying multilayer analysis to morphological, structural, and functional brain networks to identify relevant dysfunction patterns. Netw Neurosci 6(3):916–933. https://doi.org/10.1162/netn_a_00258
Shahabi H, Nair DR, Leahy RM (2023) Multilayer brain networks can identify the epileptogenic zone and seizure dynamics. Elife 17(12):e68531
Mak LE, Minuzzi L, MacQueen G, Hall G, Kennedy SH, Milev R (2017) The default mode network in healthy individuals: a systematic review and meta-analysis. Brain Connect 7(1):25–33. https://doi.org/10.1089/brain.2016.0438
Park KM, Kim KT, Kang KW, Park JA, Seo JG, Kim J, Chang H, Kim EY, Cho YW (2022) Society RLSSotKSR Alterations of functional connectivity in patients with restless legs syndrome. J Clin Neurol 18(3):290–297. https://doi.org/10.3988/jcn.2022.18.3.290
Wang Y, Li Y, Sun F, Xu Y, Xu F, Wang S, Wang X (2023) Altered neuromagnetic activity in default mode network in childhood absence epilepsy. Front Neurosci 17:1133064. https://doi.org/10.3389/fnins.2023.1133064
Malotaux V, Dricot L, Quenon L, Lhommel R, Ivanoiu A, Hanseeuw B (2023) Default-mode network connectivity changes during the progression toward Alzheimer’s dementia: a longitudinal functional magnetic resonance imaging study. Brain Connect 13(5):287–296. https://doi.org/10.1089/brain.2022.0008
Chai XJ, Ofen N, Gabrieli JD, Whitfield-Gabrieli S (2014) Development of deactivation of the default-mode network during episodic memory formation. Neuroimage 84:932–938. https://doi.org/10.1016/j.neuroimage.2013.09.032
Sestieri C, Corbetta M, Romani GL, Shulman GL (2011) Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci 31(12):4407–4420. https://doi.org/10.1523/Jneurosci.3335-10.2011
Lee DA, Lee S, Kim DW, Lee HJ, Park KM (2021) Effective connectivity alteration according to recurrence in transient global amnesia. Neuroradiology 63(9):1441–1449. https://doi.org/10.1007/s00234-021-02645-7
Funding
This work was supported by the Ministry of Science and ICT of the Republic of Korea (NRF-2021R1F1A1049605).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, D.A., Lee, HJ. & Park, K.M. Involvement of the default mode network in patients with transient global amnesia: multilayer network. Neuroradiology 65, 1729–1736 (2023). https://doi.org/10.1007/s00234-023-03241-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-023-03241-7