Abstract
Purpose
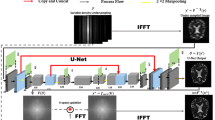
The purpose of this study is to evaluate the influence of super-resolution deep learning-based reconstruction (SR-DLR), which utilizes k-space data, on the quality of images and the quantitation of the apparent diffusion coefficient (ADC) for diffusion-weighted images (DWI) in brain magnetic resonance imaging (MRI).
Methods
A retrospective analysis was performed on 34 patients who had undergone DWI using a 3 T MRI system with SR-DLR reconstruction based on k-space data in August 2022. DWI was reconstructed with SR-DLR (Matrix = 684 × 684) and without SR-DLR (Matrix = 228 × 228). Measurements were made of the signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR) in white matter (WM) and grey matter (GM), and the full width at half maximum (FWHM) of the septum pellucidum. Two radiologists assessed image noise, contrast, artifacts, blur, and the overall quality of three image types using a four-point scale. Quantitative and qualitative scores between images with and without SR-DLR were compared using the Wilcoxon signed-rank test.
Results
Images with SR-DLR showed significantly higher SNRs and CNRs than those without SR-DLR (p < 0.001). No statistically significant variances were found in the apparent diffusion coefficients (ADCs) in WM and GM between images with and without SR-DLR (ADC in WM, p = 0.945; ADC in GM, p = 0.235). Moreover, the FWHM without SR-DLR was notably lower compared to that with SR-DLR (p < 0.001).
Conclusion
SR-DLR has the potential to augment the quality of DWI in DL MRI scans without significantly impacting ADC quantitation.






Similar content being viewed by others
Data availability
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CNR:
-
Contrast-to-noise ratio
- DLR:
-
Deep learning-based reconstruction
- DWI:
-
Diffusion-weighted imaging
- EPI:
-
Echo-planar imaging
- FWHM:
-
Full width at half maximum
- GM:
-
Grey matter
- SD:
-
Signal intensity
- SI:
-
Standard deviation
- SNR:
-
Signal-to-noise ratio
- SR-DLR:
-
Super-resolution deep learning reconstruction
- WM:
-
White matter
References
Kono K, Inoue Y, Nakayama K, Shakudo M, Morino M, Ohata K et al (2001) The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 22(6):1081–1088
Okorie CK, Ogbole GI, Owolabi MO, Ogun O, Adeyinka A, Ogunniyi A (2015) Role of diffusion-weighted imaging in acute stroke management using low-field magnetic resonance imaging in resource-limited settings. West Afr J Radiol 22(2):61–66
Butts K, Riederer SJ, Ehman RL, Thompson RM, Jack CR (1994) Interleaved echo planar imaging on a standard MRI system. Magn Reson Med 31(1):67–72
Porter DA, Heidemann RM (2009) High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med 62(2):468–475
Morelli J, Porter D, Ai F, Gerdes C, Saettele M, Feiweier T et al (2013) Clinical evaluation of single-shot and readout-segmented diffusion-weighted imaging in stroke patients at 3 T. Acta Radiol 54(3):299–306
Wang Y, Ma X, Zhang Z, Dai E, Jeong HK, Xie B et al (2018) A comparison of readout segmented EPI and interleaved EPI in high-resolution diffusion weighted imaging. Magn Reson Imaging 1(47):39–47
Kidoh M, Shinoda K, Kitajima M, Isogawa K, Nambu M, Uetani H et al (2020) Deep learning based noise reduction for brain MR imaging: tests on phantoms and healthy volunteers. Magn Reson Med Sci 19(3):195–206
Uetani H, Nakaura T, Nakaura T, Kitajima M, Morita K, Haraoka Kentaro et al (2022) Hybrid deep-learning-based denoising method for compressed sensing in pituitary MRI: comparison with the conventional wavelet-based denoising method. Eur Radiol 32(7):4527–453
Uetani H, Nakaura T, Kitajima M, Yuichi Y, Hamasaki T, Tateishi M et al (2021) A preliminary study of deep learning-based reconstruction specialized for denoising in high-frequency domain: usefulness in high-resolution three-dimensional magnetic resonance cisternography of the cerebellopontine angle. Neuroradiology 63(1):63–71
Higaki T, Nakamura Y, Tatsugami F, Nakaura T, Awai K (2019) Improvement of image quality at CT and MRI using deep learning. Jpn J Radiol 37(1):73–80
Yasaka K, Akai H, Sugawara H, Tajima T, Akahane M, Yoshioka N et al (2022) Impact of deep learning reconstruction on intracranial 1.5 T magnetic resonance angiography. Jpn J Radiol 40(5):476–83
Chaudhari AS, Fang Z, Kogan F, Wood JP, Stevens KJ, Gibbons EK et al (2018) Super-resolution musculoskeletal MRI using deep learning. Magn Reson Med 80(5):2139–2154
Chaudhari AS, Stevens KJ, Wood JP, Chakraborty Amit K, Chakraborty A, Chakraborty Amit et al (2020) Utility of deep learning super-resolution in the context of osteoarthritis MRI biomarkers. J Magn Reson Imaging. 51(3):768–79
Pham CH, Tor-Díez C, Meunier H, Bednarek N, Fablet R et al (2019) Multiscale brain MRI super-resolution using deep 3D convolutional networks. Comput Med Imaging Graph 77:101647
Du YP, Parker DL, Davis WL, Cao G (1994) Reduction of partial-volume artifacts with zero-filled interpolation in three-dimensional MR angiography. J Magn Reson Imaging 4(5):733–741
Bernstein MA, Fain SB, Riederer SJ (2001) Effect of windowing and zero-filled reconstruction of MRI data on spatial resolution and acquisition strategy. J Magn Reson Imaging 14(3):270–280
Kutsuna H, Uematsu S, Shinoda K (2023) High resolution MR reconstruction with functionally separate neural networks. ISMRM. No. 2922
Basser PJ, Mattiello J, LeBihan D (1994) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103(3):247–254
Yoshida M, Nakaura T, Inoue T, Tanoue S, Takada S, Utsunomiya D et al (2018) Magnetic resonance cholangiopancreatography with GRASE sequence at 3.0T: does it improve image quality and acquisition time as compared with 3D TSE? Eur Radiol 28(6):2436–43
Chen Z, Pawar K, Ekanayake M, Pain C, Zhong S, Egan GF (2022) Deep learning for image enhancement and correction in magnetic resonance imaging—state-of-the-art and challenges. J Digit Imaging 36(1):204–230
Zhao M, Wei Y, Wong KKL (2022) A generative adversarial network technique for high-quality super-resolution reconstruction of cardiac magnetic resonance images. Magn Reson Imaging 1(85):153–160
Chun J, Chun J, Zhang H, Gach HM, Olberg S, Mazur TR et al (2019) MRI super-resolution reconstruction for MRI-guided adaptive radiotherapy using cascaded deep learning: in the presence of limited training data and unknown translation model. Med Phys 46(9):4148–4164
Gibbs JW (1898) Fourier’s Series. Nature 59(1522):200–200
Acknowledgements
We thank Ms. Tae Hamakawa from Department of Diagnostic Radiology, Kumamoto University, Japan, for her help with the measuring in the quantitative analysis. We thank Mr. Takumi Saito from Canon Medical systems for the adjustment of SR-DLR reconstruction parameters.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest statement
Toshinori Hirai has received research support from Canon Medical Systems. Yuichi Yamahita (MRI Clinical Strategy Group Manager) and Kensuke Shinoda (Senior Engineer) are employees of Canon Medical Systems. They don’t have a fiduciary responsibility there. Canon Medical Systems had no control over the interpretation, writing, or publication of this work. Takeshi Nakaura (Corresponding author) controlled the data.
Ethics approval
This retrospective study was approved by the institutional review board (Kumamoto University, No. 1865).
Informed consent
Informed consent for this retrospective study was waived by the institutional ethics committee.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Summary
The findings from this feasibility study indicate that SR-DLR, when utilizing k-space data, may improve the quality of DWI in brain MRI while not significantly affecting the quantitation of ADCs.
Key results
1. Super-resolution deep learning-based reconstruction (SR-DLR) using k-space data significantly improved signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) in diffusion-weighted images (DWI) in brain MRI.
2. Apparent diffusion coefficient (ADC) quantitation in the white and grey matter was not significantly affected by the use of SR-DLR in DWI scans.
3. The full width at half maximum (FWHM) of the septum pellucidum was significantly reduced with the use of SR-DLR, indicating a reduction in image blur.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Matsuo, K., Nakaura, T., Morita, K. et al. Feasibility study of super-resolution deep learning-based reconstruction using k-space data in brain diffusion-weighted images. Neuroradiology 65, 1619–1629 (2023). https://doi.org/10.1007/s00234-023-03212-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-023-03212-y