Abstract
Purpose
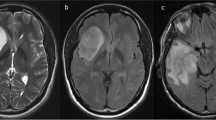
While the T2-FLAIR mismatch sign is highly specific for isocitrate dehydrogenase (IDH)-mutant, 1p/19q-noncodeleted astrocytomas among lower-grade gliomas, its utility in WHO grade 4 gliomas is not well-studied. We derived the partial T2-FLAIR mismatch sign as an imaging biomarker for IDH mutation in WHO grade 4 gliomas.
Methods
Preoperative MRI scans of adult WHO grade 4 glioma patients (n = 2165) from the multi-institutional ReSPOND (Radiomics Signatures for PrecisiON Diagnostics) consortium were analyzed. Diagnostic performance of the partial T2-FLAIR mismatch sign was evaluated. Subset analyses were performed to assess associations of imaging markers with overall survival (OS).
Results
One hundred twenty-one (5.6%) of 2165 grade 4 gliomas were IDH-mutant. Partial T2-FLAIR mismatch was present in 40 (1.8%) cases, 32 of which were IDH-mutant, yielding 26.4% sensitivity, 99.6% specificity, 80.0% positive predictive value, and 95.8% negative predictive value. Multivariate logistic regression demonstrated IDH mutation was significantly associated with partial T2-FLAIR mismatch (odds ratio [OR] 5.715, 95% CI [1.896, 17.221], p = 0.002), younger age (OR 0.911 [0.895, 0.927], p < 0.001), tumor centered in frontal lobe (OR 3.842, [2.361, 6.251], p < 0.001), absence of multicentricity (OR 0.173, [0.049, 0.612], p = 0.007), and presence of cystic (OR 6.596, [3.023, 14.391], p < 0.001) or non-enhancing solid components (OR 6.069, [3.371, 10.928], p < 0.001). Multivariate Cox analysis demonstrated cystic components (p = 0.024) and non-enhancing solid components (p = 0.003) were associated with longer OS, while older age (p < 0.001), frontal lobe center (p = 0.008), multifocality (p < 0.001), and multicentricity (p < 0.001) were associated with shorter OS.
Conclusion
Partial T2-FLAIR mismatch sign is highly specific for IDH mutation in WHO grade 4 gliomas.




Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23(8):1231–1251
Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321(5897):1807–1812
Beiko J, Suki D, Hess KR et al (2014) IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol 16(1):81–91
Patel SH, Poisson LM, Brat DJ et al (2017) T2-FLAIR mismatch, an imaging biomarker for IDH and 1p/19q status in lower-grade gliomas: a TCGA/TCIA project. Clin Cancer Res 23(20):6078–6085
Broen MPG, Smits M, Wijnenga MMJ et al (2018) The T2-FLAIR mismatch sign as an imaging marker for non-enhancing IDH-mutant, 1p/19q-intact lower-grade glioma: a validation study. Neuro Oncol 20(10):1393–1399
Jain R, Johnson DR, Patel SH et al (2020) “Real world” use of a highly reliable imaging sign: “T2-FLAIR mismatch” for identification of IDH mutant astrocytomas. Neuro Oncol 22(7):936–943
Do YA, Cho SJ, Choi BS et al (2022) Predictive accuracy of T2-FLAIR mismatch sign for the IDH-mutant, 1p/19q noncodeleted low-grade glioma: an updated systematic review and meta-analysis. Neurooncol Adv 4(1):vdac010
Han Z, Chen Q, Zhang L, et al. (2022) Radiogenomic association between the T2-FLAIR mismatch sign and IDH mutation status in adult patients with lower-grade gliomas: an updated systematic review and meta-analysis. Eur Radiol
Foltyn M, Nieto Taborda KN, Neuberger U et al (2020) T2/FLAIR-mismatch sign for noninvasive detection of IDH-mutant 1p/19q non-codeleted gliomas: validity and pathophysiology. Neurooncol Adv 2(1):vdaa004
Patel SH, Batchala PP, Muttikkal TJE et al (2021) Fluid attenuation in non-contrast-enhancing tumor (nCET): an MRI Marker for Isocitrate Dehydrogenase (IDH) mutation in Glioblastoma. J Neurooncol 152(3):523–531
Davatzikos C, Barnholtz-Sloan JS, Bakas S et al (2020) AI-based prognostic imaging biomarkers for precision neuro-oncology: the ReSPOND consortium. Neuro Oncol 22(6):886–888
Menze BH, Jakab A, Bauer S et al (2015) The multimodal brain tumor image segmentation benchmark (BRATS). IEEE Trans Med Imaging 34(10):1993–2024
Pati S, Baid U, Edwards B et al (2022) Federated learning enables big data for rare cancer boundary detection. Nat Commun 13(1):7346
Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A (2010) The SRI24 multichannel atlas of normal adult human brain structure. Hum Brain Mapp 31(5):798–819
Lasocki A, Gaillard F (2019) Non-contrast-enhancing tumor: a new frontier in glioblastoma research. AJNR Am J Neuroradiol 40(5):758–765
VASARI Research Project. https://wiki.cancerimagingarchive.net/display/Public/VASARI+Research+Project. Accessed 8/17/2022.
Chang K, Bai HX, Zhou H et al (2018) Residual convolutional neural network for the determination of IDH status in low- and high-grade gliomas from MR imaging. Clin Cancer Res 24(5):1073–1081
Choi YS, Bae S, Chang JH et al (2021) Fully automated hybrid approach to predict the IDH mutation status of gliomas via deep learning and radiomics. Neuro Oncol 23(2):304–313
Jian A, Jang K, Manuguerra M, Liu S, Magnussen J, Di Ieva A (2021) Machine learning for the prediction of molecular markers in glioma on magnetic resonance imaging: a systematic review and meta-analysis. Neurosurgery 89(1):31–44
Calabrese E, Rudie JD, Rauschecker AM et al (2022) Combining radiomics and deep convolutional neural network features from preoperative MRI for predicting clinically relevant genetic biomarkers in glioblastoma. Neurooncol Adv 4(1):vdac060
Mohammed S, Ravikumar V, Warner E et al (2022) Quantifying T2-FLAIR mismatch using geographically weighted regression and predicting molecular status in lower-grade gliomas. AJNR Am J Neuroradiol 43(1):33–39
Pope WBSJ, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF (2005) MR Imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol 26:2466–2474
Lasocki A, Gaillard F, Tacey M, Drummond K, Stuckey S (2016) Incidence and prognostic significance of non-enhancing cortical signal abnormality in glioblastoma. J Med Imaging Radiat Oncol 60(1):66–73
Carrillo JA, Lai A, Nghiemphu PL et al (2012) Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am J Neuroradiol 33(7):1349–1355
Han S, Liu Y, Cai SJ et al (2020) IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer 122(11):1580–1589
Deguchi S, Oishi T, Mitsuya K et al (2020) Clinicopathological analysis of T2-FLAIR mismatch sign in lower-grade gliomas. Sci Rep 10(1):10113
Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ (2018) Clinically relevant imaging features for MGMT promoter methylation in multiple glioblastoma studies: a systematic review and meta-analysis. AJNR Am J Neuroradiol 39(8):1439–1445
Yogananda CGB, Shah BR, Nalawade SS et al (2021) MRI-based deep-learning method for determining glioma MGMT promoter methylation status. AJNR Am J Neuroradiol 42(5):845–852
Kinoshita M, Arita H, Takahashi M et al (2020) Impact of inversion time for FLAIR acquisition on the T2-FLAIR mismatch detectability for IDH-mutant, non-CODEL astrocytomas. Front Oncol 10:596448
Acknowledgements
This study was previously presented as a poster at the Society for Neuro-Oncology annual meeting in Tampa, Florida on November 18, 2022, and as an oral presentation at the Radiological Society of North America annual meeting in Chicago, Illinois on November 27, 2022.
The ReSPOND Consortium also includes: Stephen J. Bagley1,2, Michel Bilello3,4, Steven Brem5,1, Ujjwal Baid3,6, Arati S. Desai1,2, Robert A. Lustig7, Elizabeth Mamourian3,6, Anahita Fathi Kazerooni8,9,3, Jose A. Garcia3,6, Donald M. O’Rourke5,1, Zev A. Binder1, Mikhail Milchenko10, Arash Nazeri10, Aris Sotiras10, Murat Ak11, Jaume Capellades12, Josep Puig13, Sung Soo Ahn14, Jong Hee Chang15,16, Seung-Koo Lee14, Yae Won Park14, Vachan Vadmal17, Kristin A. Waite18, Sree Gongala19, Alysha Chelliah20, Golestan Karami20, Gregory S. Alexander21, Ayesha S. Ali22, Spencer Liem22, Joseph Lombardo22,23, Gaurav Shukla22,24,3, Muhammad Sharif22, Lisa R. Rogers25, William Taylor26, Santiago Cepeda27, Aikaterini Kotrotsou28, Hassan Fathallah-Shaykh29, Orazio Santo Santonocito30, Anna Luisa Di Stefano30, Aaron M. Rulseh31, Yuji Matsumoto32, Kimberley Alexander33,34,35, Laveniya Satgunaseelan36, Benedikt Wiestler37, Rao P. Gullapalli38, Elias R. Melhem38, Graeme F. Woodworth38,39, Peter I. Kamel40, Victor M. Perez-Garcia41, Alekos Vamvakas42, Yiannis Tsougos42, Pablo Valdes43, Pallavi Tiwari44, Mariam Aboian45,46,47
1Glioblastoma Multiforme Translational Center of Excellence, Abramson Cancer Center, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA
2Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA
3Center for Biomedical Image Computing and Analytics (CBICA), University of Pennsylvania, Philadelphia, PA, USA
4Department of Radiology, Division of Neuroradiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA
5Department of Neurosurgery, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA
6Department of Radiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA
7Department of Radiation Oncology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA
8Center for Data Driven Discovery in Biomedicine, Children’s Hospital of Philadelphia, Philadelphia, PA, USA
9Center for AI and Data Science for Integrated Diagnostics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, USA
10Department of Radiology, Washington University School of Medicine, St. Louis, MO, USA
11Department of Radiology, University of Pittsburgh, Pittsburgh, PA, USA
12Department of Medical Imaging, Consorci MAR Parc de Salut, Barcelona, Spain
13Research Unit (IDIR) Image Diagnosis Institute, Badalona, Spain
14Department of Radiology, Section of Neuroradiology, Yonsei University Health System, Seoul, South Korea
15Department of Neurosurgery, Yonsei University College of Medicine, Seoul, South Korea
16Brain Tumor Center, Severance Hospital, Yonsei University Health System, Seoul, South Korea
17Department of Population and Quantitative Health Sciences, Case Western Reserve University and University Hospitals of Cleveland, Cleveland, OH, USA
18Trans-Divisional Research Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA
19Department of Radiology, Case Western Reserve University and University Hospitals of Cleveland, Cleveland, OH, USA
20School of Biomedical Engineering and Imaging Sciences, King’s College London, London, UK
21Department of Radiation Oncology, University of Maryland, Baltimore, MD, USA
22Department of Radiation Oncology, Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA, USA
23Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, PA, USA
24Department of Radiation Oncology, Christiana Care Health System, Philadelphia, PA, USA
25Department of Neurosurgery, Hermelin Brain Tumor Center, Henry Ford Cancer Institute, Henry Ford Health, Detroit, MI, USA
26Department of Radiation Oncology and Neurosurgery, The James Cancer Hospital at the Ohio State University Wexner Medical Center, Columbus, OH, USA
27Department of Neurosurgery, University Hospital Río Hortega, Valladolid, Spain
28MD Anderson Cancer Center, University of Texas, Houston, TX, USA
29Department of Neurology, The University of Alabama at Birmingham, Birmingham, AL, USA
30Division of Neurosurgery, Spedali Riuniti di Livorno-Azienda USL Toscana Nord-Ovest, Livorno, Italy
31Department of Radiology, Na Homolce Hospital, Prague, Czech Republic
32Department of Neurological Surgery, Okayama University, Okayama, Japan
33Chris O’Brien Lifehouse, Camperdown, Australia
34University of Sydney, Camperdown, Australia
35Sydney Local Health District, Camperdown, Australia
36Department of Pathology Services, Royal Prince Alfred Hospital, Camperdown, Australia
37Department of Neuroradiology, Technical University of Munich, Munchen, Germany
38Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, USA
39Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, MD, USA
40Department of Diagnostic Radiology and Nuclear Medicine, Intelligent Imaging (UM2ii) Center, University of Maryland School of Medicine, Baltimore, MD, USA
41Mathematical Oncology Laboratory (MOLAB), University of Castilla-La Mancha, Ciudad Real, Spain
42Medical School of the University of Thessaly, Larissa, Greece
43University of Texas Medical Branch, Galveston, TX, USA
44Department of Radiology and Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, USA
45Clinical Advanced Image Processing Lab (CAIP), Yale School of Medicine, New Haven, CT, USA
46Brain Tumor Research Group (ImagineQuant), Yale School of Medicine, New Haven, CT, USA
47Section of Neuroradiology and Nuclear Medicine, Yale School of Medicine, New Haven, CT, USA
ORCIDs: Stephen J. Bagley, https://orcid.org/0000-0002-7117-0539; Michel Bilello, https://orcid.org/0000-0001-6313-5437; Steven Brem, https://orcid.org/0000-0002-5803-8920; Ujjwal Baid, https://orcid.org/0000-0001-5246-2088; Arati S. Desai, https://orcid.org/0000-0002-4849-4703; Robert A. Lustig, https://orcid.org/0000-0003-0633-3802; Elizabeth Mamourian, http://orcid.org/0000-0001-8581-4887; Anahita Fathi Kazerooni, https://orcid.org/0000-0001-7131-2261; Donald M. O’Rourke, https://orcid.org/0000-0002-8479-7314; Zev A. Binder, https://orcid.org/0000-0003-1158-231X; Mikhail Milchenko, https://orcid.org/0000-0002-4022-1081; Arash Nazeri, https://orcid.org/0000-0001-6983-0641; Aris Sotiras, https://orcid.org/0000-0003-0795-8820; Murat Ak, https://orcid.org/0000-0001-7384-478X; Jaume Capellades, https://orcid.org/0000-0002-1417-4496; Josep Puig, https://orcid.org/0000-0003-2791-6599; Sung Soo Ahn, https://orcid.org/0000-0002-0503-5558; Jong Hee Chang, https://orcid.org/0000-0003-1509-9800; Seung-Koo Lee, https://orcid.org/0000-0001-5646-4072; Yae Won Park, https://orcid.org/0000-0001-8907-5401; Kristin A. Waite, https://orcid.org/0000-0002-3186-8510; Alysha Chelliah, https://orcid.org/0000-0003-0867-1565; Golestan Karami, https://orcid.org/0000-0002-8107-3812; Gregory S. Alexander, http://orcid.org/0000-0003-1907-7828; Santiago Cepeda, https://orcid.org/0000-0003-1667-8548; Aikaterini Kotrotsou, https://orcid.org/0000-0002-0433-7159; Hassan Fathallah-Shaykh, https://orcid.org/0000-0002-2690-7685; Orazio Santo Santonocito, https://orcid.org/0000-0002-1071-7166; Anna Luisa Di Stefano, https://orcid.org/0000-0003-1746-0647; Aaron M. Rulseh, https://orcid.org/0000-0002-8332-4419; Yuji Matsumoto, https://orcid.org/0000-0001-8798-381X; Kimberley Alexander, https://orcid.org/0000-0002-7239-039X; Laveniya Satgunaseelan, https://orcid.org/0000-0002-7435-0834; Benedikt Wiestler, https://orcid.org/0000-0002-2963-7772; Rao P. Gullapalli, https://orcid.org/0000-0003-0551-0379; Victor M. Perez-Garcia, https://orcid.org/0000-0002-6575-495X; Yiannis Tsougos, https://orcid.org/0000-0002-5204-5273; Pallavi Tiwari, https://orcid.org/0000-0001-9477-4856; Mariam Aboian, https://orcid.org/0000-0002-4877-8271
Funding
This project was partially supported by National Institutes of Health/National Cancer Institute (R01CA269948).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the appropriate institutional research boards.
Informed consent
Informed consent was waived.
Conflict of interest
The authors declare no that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, M.D., Patel, S.H., Mohan, S. et al. Association of partial T2-FLAIR mismatch sign and isocitrate dehydrogenase mutation in WHO grade 4 gliomas: results from the ReSPOND consortium. Neuroradiology 65, 1343–1352 (2023). https://doi.org/10.1007/s00234-023-03196-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-023-03196-9