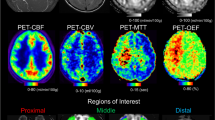
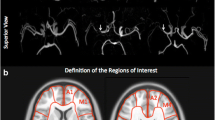
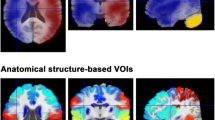
Abstract
Purpose
Accurate assessment of cerebral perfusion in moyamoya disease is necessary to determine the indication for treatment. We aimed to investigate the usefulness of dynamic PCASL using a variable TR scheme with optimized background suppression in the evaluation of cerebral perfusion in moyamoya disease.
Methods
We retrospectively analyzed the images of 24 patients (6 men and 18 women, mean age 31.4 ± 18.2 years) with moyamoya disease; each of whom was imaged with both dynamic PCASL using the variable-TR scheme and 123IMP SPECT with acetazolamide challenge. ASL dynamic data at 10 phases are acquired by changing the LD and PLD. The background suppression timing was optimized for each phase. CBF and ATT were measured with ASL, and CBF and CVR to an acetazolamide challenge were measured with SPECT.
Results
A significant moderate correlation was found between the CBF measured by dynamic PCASL and that by SPECT (r = 0.53, P < 0.001). The CBF measured by dynamic PCASL (52.5 ± 13.3 ml/100 mg/min) was significantly higher than that measured by SPECT (43.0 ± 12.6 ml/100 mg/min, P < 0.001). The ATT measured by dynamic PCASL showed a significant correlation with the CVR measured by SPECT (r = 0.44, P < 0.001). ATT was significantly longer in areas where the CVR was impaired (CVR < 18.4%, ATT = 1812 ± 353 ms) than in areas where it was preserved (CVR > 18.4%, ATT = 1301 ± 437 ms, P < 0.001). The ROC analysis showed a moderate accuracy (AUC = 0.807, sensitivity = 87.7%, specificity = 70.4%) when the cutoff value of ATT was set at 1518 ms.
Conclusion
Dynamic PCASL using this scheme was found to be useful for assessing cerebral perfusion in moyamoya disease.






Similar content being viewed by others
Data availability
The data will be made available by the corresponding author upon reasonable request. Anonymized data will be shared by request from qualified investigators.
Abbreviations
- ASL:
-
Arterial spin labeling
- AUC:
-
Area under the curve
- ATT:
-
Arterial transit time
- CBF:
-
Cerebral blood flow
- CVR:
-
Cerebrovascular reactivity
- 123I-IMP:
-
Iodine-123-N-isopropyl-p-iodoamphetamine
- LD:
-
Label duration
- PCASL:
-
Pseudo-continuous arterial spin labeling
- PLD:
-
Post-labeling delay
- SPECT:
-
Single photon emission computed tomography
- TR:
-
Repetition time
References
Ikezaki K, Matsushima T, Kuwabara Y, Suzuki SO, Nomura T, Fukui M (1994) Cerebral circulation and oxygen metabolism in childhood moyamoya disease: a perioperative positron emission tomography study. J Neurosurg 81(6):843–850. https://doi.org/10.3171/jns.1994.81.6.0843
Kuwabara Y, Ichiya Y, Sasaki M, Yoshida T, Masuda K, Ikezaki K, Matsushima T, Fukui M (1997) Cerebral hemodynamics and metabolism in moyamoya disease–a positron emission tomography study. Clin Neurol Neurosurg 99(Suppl 2):S74-78. https://doi.org/10.1016/s0303-8467(97)00061-9
Tominaga T, Suzuki N, Miyamoto S, Koizumi A, Kuroda S, Takahashi J, Fujimura M, Houkin K (2018) Recommendations for the management of moyamoya disease: a statement from research committee on spontaneous occlusion of the circle of Willis (moyamoya disease) [2nd edition]. Surg Cerebral Stroke 46(1):1–24. https://doi.org/10.2335/scs.46.1
Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73(1):102–116. https://doi.org/10.1002/mrm.25197
Setta K, Matsuda T, Sasaki M, Chiba T, Fujiwara S, Kobayashi M, Yoshida K, Kubo Y, Suzuki M, Yoshioka K, Ogasawara K (2021) Diagnostic accuracy of screening arterial spin-labeling MRI using Hadamard encoding for the detection of reduced CBF in adult patients with ischemic moyamoya disease. AJNR Am J Neuroradiol 42(8):1403–1409. https://doi.org/10.3174/ajnr.A7167
Obara M, Togao O, Wada T, Tokunaga C, Mikayama R, Hamano H, van de Ven K, Yoneyama M, Ogino T, Akamine Y, Ueda Y, Kwon J, Van Cauteren M (2021) Pseudo-continuous arterial spin labeling using multiple label- and post-label duration with dynamically optimized background suppression. Proc Int Soc Magn Reson Med 870
Nishizawa S, Iida H, Tsuchida T, Ito H, Konishi J, Yonekura Y (2003) Validation of the dual-table autoradiographic method to quantify two sequential rCBFs in a single SPET session with N-isopropyl-[123I] p-iodoamphetamine. Eur J Nucl Med Mol Imaging 30(7):943–950. https://doi.org/10.1007/s00259-003-1180-7
Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR (1998) A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med 40(3):383–396. https://doi.org/10.1002/mrm.1910400308
Inokuchi Y, Koya S, Uematsu M, Takashina T (2019) Usefulness of the frontal lobe bottom and cerebellum tuber vermis line as an alternative clue to set the axial angle parallel to the AC-PC line in I-123 IMP SPECT imaging: a retrospective study. Radiol Phys Technol 12(4):388–392. https://doi.org/10.1007/s12194-019-00535-5
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15(2):155–163. https://doi.org/10.1016/j.jcm.2016.02.012
Ogasawara K, Ito H, Sasoh M, Okuguchi T, Kobayashi M, Yukawa H, Terasaki K, Ogawa A (2003) Quantitative measurement of regional cerebrovascular reactivity to acetazolamide using 123I-N-isopropyl-p-iodoamphetamine autoradiography with SPECT: validation study using H2 15O with PET. J Nucl Med 44(4):520–525
Yun TJ, Sohn CH, Han MH, Kang HS, Kim JE, Yoon BW, Paeng JC, Choi SH, Kim JH, Song IC, Chang KH (2013) Effect of delayed transit time on arterial spin labeling: correlation with dynamic susceptibility contrast perfusion magnetic resonance in moyamoya disease. Invest Radiol 48(11):795–802. https://doi.org/10.1097/RLI.0b013e3182981137
Ha JY, Choi YH, Lee S, Cho YJ, Cheon JE, Kim IO, Kim WS (2019) Arterial spin labeling MRI for quantitative assessment of cerebral perfusion before and after cerebral revascularization in children with moyamoya disease. Korean J Radiol : Off J Korean Radiol Soc 20(6):985–996. https://doi.org/10.3348/kjr.2018.0651
Lee S, Yun TJ, Yoo RE, Yoon BW, Kang KM, Choi SH, Kim JH, Kim JE, Sohn CH, Han MH (2018) Monitoring cerebral perfusion changes after revascularization in patients with moyamoya disease by using arterial spin-labeling MR imaging. Radiol 288(2):565–572. https://doi.org/10.1148/radiol.2018170509
Tortora D, Scavetta C, Rebella G, Bertamino M, Scala M, Giacomini T, Morana G, Pavanello M, Rossi A, Severino M (2020) Spatial coefficient of variation applied to arterial spin labeling MRI may contribute to predict surgical revascularization outcomes in pediatric moyamoya vasculopathy. Neuroradiol 62(8):1003–1015. https://doi.org/10.1007/s00234-020-02446-4
Hara S, Tanaka Y, Ueda Y, Hayashi S, Inaji M, Ishiwata K, Ishii K, Maehara T, Nariai T (2017) Noninvasive evaluation of CBF and perfusion delay of moyamoya disease using arterial spin-labeling MRI with multiple postlabeling delays: comparison with (15)O-gas PET and DSC-MRI. AJNR Am J Neuroradiol 38(4):696–702. https://doi.org/10.3174/ajnr.A5068
Kim JH, Lee SJ, Shin T, Kang KH, Choi PY, Kim JH, Gong JC, Choi NC, Lim BH (2000) Correlative assessment of hemodynamic parameters obtained with T2*-weighted perfusion MR imaging and SPECT in symptomatic carotid artery occlusion. AJNR Am J Neuroradiol 21(8):1450–1456
Kikuchi K, Murase K, Miki H, Yasuhara Y, Sugawara Y, Mochizuki T, Ikezoe J, Ohue S (2002) Quantitative evaluation of mean transit times obtained with dynamic susceptibility contrast-enhanced MR imaging and with (133)Xe SPECT in occlusive cerebrovascular disease. AJR Am J Roentgenol 179(1):229–235. https://doi.org/10.2214/ajr.179.1.1790229
Gibbs JM, Wise RJ, Leenders KL, Jones T (1984) Evaluation of cerebral perfusion reserve in patients with carotid-artery occlusion. Lancet 1(8372):310–314. https://doi.org/10.1016/s0140-6736(84)90361-1
Schumann P, Touzani O, Young AR, Morello R, Baron JC, MacKenzie ET (1998) Evaluation of the ratio of cerebral blood flow to cerebral blood volume as an index of local cerebral perfusion pressure. Brain 121(Pt 7):1369–1379. https://doi.org/10.1093/brain/121.7.1369
Vagal AS, Leach JL, Fernandez-Ulloa M, Zuccarello M (2009) The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. AJNR Am J Neuroradiol 30(5):876–884. https://doi.org/10.3174/ajnr.A1538
Kawano T, Ohmori Y, Kaku Y, Muta D, Uekawa K, Nakagawa T, Amadatsu T, Kasamo D, Shiraishi S, Kitajima M, Kuratsu J (2016) Prolonged mean transit time detected by dynamic susceptibility contrast magnetic resonance imaging predicts cerebrovascular reserve impairment in patients with woyamoya disease. Cerebrovasc Dis 42(1–2):131–138. https://doi.org/10.1159/000445696
Wang R, Yu S, Alger JR, Zuo Z, Chen J, Wang R, An J, Wang B, Zhao J, Xue R, Wang DJ (2014) Multi-delay arterial spin labeling perfusion MRI in moyamoya disease–comparison with CT perfusion imaging. Eur Radiol 24(5):1135–1144. https://doi.org/10.1007/s00330-014-3098-9
Choi HJ, Sohn CH, You SH, Yoo RE, Kang KM, Yun TJ, Choi SH, Kim JH, Cho WS, Kim JE (2018) Can arterial spin-labeling with multiple postlabeling delays predict cerebrovascular reserve? AJNR Am J Neuroradiol 39(1):84–90. https://doi.org/10.3174/ajnr.A5439
Inugami A, Kanno I, Uemura K, Shishido F, Murakami M, Tomura N, Fujita H, Higano S (1988) Linearization correction of 99mTc-labeled hexamethyl-propylene amine oxime (HM-PAO) image in terms of regional CBF distribution: comparison to C15O2 inhalation steady-state method measured by positron emission tomography. J Cerebral Blood Flow Metab : Off J Int Soc Cerebr Blood Flow Metab 8(6):S52-60. https://doi.org/10.1038/jcbfm.1988.33
Funding
This study was supported by JSPS KAKENHI Grant Number JP20K08111.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Drs. Makoto Obara, Hiroshi Hamano, and Marc van Cauteren are employees of Philips Japan. The other authors declare no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This retrospective investigation was approved by the Institutional Review Board of Kyushu University Hospital (No. 22049–00). The informed consent requirement was waived based on the retrospective study design.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Togao, O., Obara, M., Yamashita, K. et al. Assessment of cerebral perfusion in moyamoya disease with dynamic pseudo-continuous arterial spin labeling using a variable repetition time scheme with optimized background suppression. Neuroradiology 65, 529–538 (2023). https://doi.org/10.1007/s00234-022-03095-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03095-5