Abstract
Purpose
To compare resting-state functional connectivity (RSFC) of obese patients responders or non-responders to sleeve gastrectomy (SG) with a group of obese patients with no past medical history of metabolic or bariatric surgery.
Methods
MR images were acquired at 1.5 Tesla. Resting-state fMRI data were analyzed with statistical significance threshold set at p < 0.05, family-wise error (FWE) corrected.
Results
Sixty-two subjects were enrolled: 20 controls (age range 25–64; 14 females), 24 responders (excess weight loss > 50%; age range 23–68; 17 females), and 18 non-responders to sleeve gastrectomy (SG) (excess weight loss < 50%; age range 23–67; 13 females). About within-network RSFC, responders showed significantly lower RSFC with respect to both controls and non-responders in the default mode and frontoparietal networks, positively correlating with psychological scores. Non-responders showed significantly higher (p < 0.05, family-wise error (few) corrected) RSFC in regions of the lateral visual network as compared to controls. Regarding between-network RSFC, responders showed significantly higher anti-correlation between executive control and salience networks (p < 0.05, FWE corrected) with respect to both controls and non-responders. Significant positive correlation (Spearman rho = 0.48, p = 0.0012) was found between % of excess weight loss and executive control-salience network RSFC.
Conclusion
There are differences in brain functional connectivity in either responders or non-responders patients to SG. The present results offer new insights into the neural correlates of outcome in patients who undergo SG and expand knowledge about neural mechanisms which may be related to surgical response.
Similar content being viewed by others
Introduction
Obesity is a serious public health concern that is rapidly growing over the recent past [1]. Social habits promoting the combination of inactivity and food consumption have been pointed out as major factors explaining the obesity pandemic [2, 3].
Weight loss is the most important target in obese patients. To this end, both metabolic and bariatric surgeries have been established as the most effective intervention to obtain significant and long-term weight loss in eligible patients [4, 5]. Beyond weight loss, multiple additional benefits have been associated with metabolic and bariatric surgery, including improvement of obesity-related comorbidities such as type 2 diabetes mellitus and hyperlipidemia [2, 4, 5].
Among variable surgical options, sleeve gastrectomy (SG) has recently been adopted as one of the most common surgical procedures performed for bariatric purposes [4]. Despite SG being considered a safe and effective procedure, up to 30% of the patients who had undergone SG are non-responders and necessitate further surgery due to insufficient (< 50%) excess weight loss (EWL) or weight regain [6].
The reasons why some patients do not respond to SG are not fully understood and are the subject of active research. It is likely due to a multifactorial process in which an abnormal function of the brain might be implicated [1,2,3,4,5,6].
Functional MRI (fMRI) is a non-invasive technique allowing for the analysis of brain functional connectivity (FC) in vivo thanks to the blood oxygen level–dependent (BOLD) effect, which is based on variations of magnetic susceptibility due to local changes of deoxyhemoglobin concentration [7,8,9,10]. Indeed, the temporal coupling of BOLD signal among brain regions is indicative of their FC.
fMRI can be acquired in resting-state (rs) fMRI and during stimulus or task, and it is highly sensitive in capturing small changes of brain functional connectivity [7, 10,11,12].
fMRI has been applied to investigate obese patients and showed several interesting changes of brain dynamics including positive association between regions of the reward circuit and obesity phenotype, significant differences of between-network connectivity between obesity-prone and obesity-resistant patients in the basal ganglia and right dorsolateral prefrontal cortex, and disrupted functional connectivity in adolescent between reward, emotional memories, and cognitive control [13,14,15].
Brain resting-state functional connectivity (RSFC) has been poorly explored in patients after bariatric surgery. Potential differences between patients responders (i.e., EWL > 50%) and non-responders (i.e., EWL < 50%) to SG are currently unknown.
The hypothesis behind this study is that there are differences of brain connectivity between patients who respond or not to SG, and these differences can be captured with rsfMRI.
The major aim of this study was to compare RSFC of obese patient responders or non-responders to SG with a group of obese patients with no past medical history of metabolic or bariatric surgery (i.e., control group). An additional aim was to explore correlations of brain RSFC with the amount of weight loss and measures of psychological domains.
Materials and methods
Study design and patients
This study was approved by the Ethical Committee of our institution (30/20 PAR ComEt CBM) and was designed as prospective case–control. All procedures agreed with the ethical standards of the Declaration of Helsinki, and written informed consent was obtained from all participants.
Enrolled patients were divided into three groups:
-
1.
Control group: obesity and no past medical history of metabolic or bariatric surgery
-
2.
Responders: history of laparoscopic SG at least 24 months before inclusion and EWL > 50%
-
3.
Non-responders: history of laparoscopic SG at least 24 months before inclusion and EWL < 50%
Inclusion criteria for all the groups were: (1) age > 18 years, (2) right-handedness according to the Edinburgh inventory [16], (3) minimum follow-up of 24 months with respect to surgery, (4) absence of neurological symptoms at the time of inclusion, (5) less than two white matter hyperintensities on T2 weighted FLAIR images with maximum diameter < 5 mm.
Exclusion criteria for all the groups were (1) known neurological or psychiatric disorders; (2) vascular, neoplastic, or traumatic lesions of the central nervous system; (3) history of brain irradiation or neurosurgery; (4) absolute MRI contraindications; (5) poor quality of images due to artifacts.
All the enrolled patients underwent psychological assessment and unenhanced MRI scan of the brain.
Patients who had undergone SG were evaluated by a multidisciplinary team to verify the indication to SG. All the subjects of the control group were considered eligible for SG after the evaluation of a multidisciplinary team.
The %EWL was calculated according to the following formula: %EWL = 100% * [body weight loss / (pre-surgery body weight − ideal body weight)].
Psychological assessment
Multiple emotional and behavioral domains were explored. Specifically, mental distress, emotional self-regulation mechanisms, and eating behavior were explored with self-assessment questionnaires, including the following:
-
Symptom Checklist-90 Revised: consisting of 90 items evaluating the presence and severity of symptoms of mental distress (somatization, obsession-compulsion, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, psychoticism) [17]
-
Barratt Impulsiveness Scale: a questionnaire that measures impulsiveness, specifically motor impulsivity (acting without thinking), cognitive impulsivity (making quick decisions and lack of focus on the task), and unplanned impulsivity (poor assessment of consequences and lack of planning) [18]
-
Difficulties in Emotion Regulation: a questionnaire that investigates the difficulty in regulating emotions [19]
-
Binge Eating Scale: evaluates behaviors, sensations, and cognitive aspects associated with bulimic episodes [20]
Brain MRI protocol
MR images data were acquired with the 1.5 Tesla Magnetom Aera scanner (Siemens; Erlangen, Germany), equipped with a 12-element designed head matrix coil. MRI anatomical protocol included the following sequences: 3D-MPRAGE T1-weighted and 2D fluid attenuated inversion recovery (FLAIR) T2-weighted sequence (please see Supplementary Material for details).
Whole-brain rsfMRI (230 volumes, 9 min 51 s) was achieved with echo-planar imaging with prospective acquisition correction, 76 contiguous axial slices parallel to the anterior–posterior commissure plane with interleaved multislice T2* echo-planar imaging (TR = 2500 ms, TE = 59 ms, FOV = 28 × 28 cm, flip angle = 84°, voxel size = 2.2 × 2.2 × 2.2 mm, acceleration iPAT = 4). Field mapping was obtained using a double echo GRE T2* weighted sequence (TR = 586 ms, TE1/TE2 = 4.76/9.52 ms, FOV = 19 × 19 cm, flip angle = 60°, voxel size = 3 × 3 × 3 mm).
For a description of the MRI protocol, please see Supplementary Material.
Brain MRI analysis
Anatomical and functional preprocessing were performed using fMRIPrep 20.1.1 [21] (RRID:SCR_016216), which is based on Nipype 1.5.0 [22] (RRID:SCR_002502).
For a detailed description of anatomical and functional data preprocessing, please see Supplementary Material.
MELODIC
Independent component analysis (ICA) of preprocessed functional data was performed using FSL’s MELODIC tool (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) [23]. For group-wise ICA, a single 4D dataset was created by temporally concatenating preprocessed functional data containing 230 time points for each subject. The dimensionality of group-ICA was performed using a dimensionality of 20 components [24, 25]. Resting-state networks (RSNs) of interest were identified via spatial correlation coefficients (fslcc tool) using RSNs generated by Smith et al. and Yeo et al. templates, and then verified by expert visual inspection (C.P., 10 years of experience and N.P., 17 years of experience) [12, 24,25,26].
The set of spatial maps from the group-average analysis was used to generate subject-specific versions of the spatial maps and associated time series using a dual regression technique [27, 28]. For each subject, the group-average set of spatial maps was first regressed (as spatial regressors in a multiple regression) into the subject’s 4D space–time dataset, resulting in a set of subject-specific time series, one per group-level spatial map. These time series were then regressed (as temporal regressors in a multiple regression) into the same 4D dataset, resulting in a set of subject-specific spatial maps, one per group-level spatial map.
Within-network RSFC
Subject-specific spatial maps obtained from dual regression analysis were entered into group-level voxel-wise analyses. For each selected resting-state network (RSN), FC differences between pairs of groups (i.e., controls vs responders, controls vs non-responders and responders vs non-responders) were assessed by means of two-sample unpaired t-test using general linear model implemented in FSL. Age, sex, and total intracranial volume (TIV) were entered into the model as covariates of no interest. The statistical analyses were performed by using nonparametric permutation testing (FSL Randomise, 5000 permutations) [29]. Clusters were determined by using threshold‐free cluster enhancement (TFCE) [24] and a family‐wise error (FWE)-corrected cluster significance threshold of p = 0.05. Nonparametric tests were used to safeguard against the possibility that the between‐subjects effects were non‐Gaussian and because such nonparametric inference has greater robustness against spatial non-stationarity than commonly used parametric methods [30].
Possible correlations among brain areas displaying significant between-group differences and clinical/psychological scores BES (Binge Eating Scale), SOM (somatization), OBS\COMP (obsessive–compulsive), IS (interpersonal sensitivity), PAR (paranoid thought), GSI (General Symptom Index), and %EWL for responders and non-responders only were assessed by using regression analyses (Randomise, 5000 permutations). Resulting statistical maps were thresholded at p < 0.05, FWE corrected. Anatomical localization of significant clusters was established according to the Harvard–Oxford Cortical Structural Atlas included in FSL (http://www.fmrib.ox.ac.uk/fsl/data/atlasdescriptions.html).
Between-network RSFC
Between-network RSFC differences were investigated using FSLNets toolbox (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLNets). After normalization of the extracted time courses (output of the first stage of dual regression) of all resting-state networks (RSNs) identified in each subject, time courses of artifactual components and components of no interest were regressed out of the individual data. Subject-wise correlation matrices of both full and partial correlation of all remaining RSN time courses were then created. The resulting correlation coefficients (connection or edge strengths) were then Fisher z-transformed and corrected for temporal autocorrelation. Between-subject testing was then conducted across correlation values acquired for pairs of independent components.
Between-group comparisons of time series correlations were performed using non-parametric unpaired testing (FSL Randomise tool, 5000 permutations; age, sex, and TIV as nuisance variables). The statistical significance threshold was set at p < 0.05, FWE corrected. Finally, the relationship between clinical and psychological scores and between-network RSFC was assessed using Spearman’s rank correlation, with p < 0.05 for null hypothesis rejection. The clinical and psychological variables that demonstrated significant differences among groups were used for correlation analyses.
Results
Enrolled patients
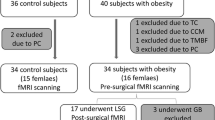
Only one patient was excluded after brain MRI due to congenital hydrocephalus. Then, a total of 62 subjects were enrolled in this study, classified as follows:
-
1.
Control group: 20 patients (age range 25–64; 14 females and 6 males)
-
2.
Responders: 24 patients (age range 23–68; 17 females and 7 males)
-
3.
Non-responders: 18 patients (age range 23–67; 13 females and 5 males)
The groups were age- and gender-matched. A more detailed description of demographic, clinical, and significant psychological features of obese patients is given in Table 1.
Within-network RSFC
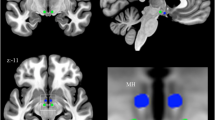
Independent component analysis (ICA) yielded 20 independent components representing group-averaged networks of brain regions with BOLD fMRI signals that were temporally correlated. Of these, we identified 9 components that showed the highest spatial correlation coefficients with RSN templates: default mode (r = 0.74), dorsal attention (r = 0.52), left and right frontoparietal (r = 0.69 and r = 0.64, respectively), executive control (r = 0.56), lateral visual (r = 0.54), medial visual (r = 0.76), cerebellar (r = 0.35), and sensorimotor (r = 0.56) networks. The salience network was instead identified according to previous literature [31, 32] (Fig. 1).
Responders showed significantly lower (p < 0.05, FWE corrected) within-network RSFC in respect to both controls and non-responders in several regions of the default mode and frontoparietal networks (Fig. 2). Regarding the default mode network, responders showed lower RSFC with respect to controls in the anterior regions, while they showed lower RSFC as compared to non-responders in the posterior core regions (precuneus/posterior cingulate gyrus) of the network.
Within-network FC. Significantly higher within-network FC in a controls with respect to responders, b non-responders with respect to responders, and c non-responders with respect to controls. (p < 0.05, FWE corrected). Results for each RSN are overlaid onto the corresponding network (green) in the MNI152 standard brain. The red-yellow color bar represents the level of significance
Regarding frontoparietal networks, responders showed lower RSFC with respect to controls in both anterior and posterior regions of the right frontoparietal network, while they showed lower RSFC with respect to non-responders only in the anterior regions (see Tables 2 and 3). Furthermore, the comparison between the two patient groups showed a significant difference in the posterior regions of the left frontoparietal network, with the responders showing lower RSFC in the supramarginal and angular gyri.
We did not find significant RSFC differences between controls and non-responders in these cognitive RSNs. However, we found significantly higher RSFC in non-responders with respect to controls in regions of the lateral visual network, such as bilateral occipital poles and the inferior division of the lateral occipital cortices (see Table 4).
Since behavioral differences in clinical scores were only found between controls and responders, correlation analyses between clinical scores and RSFC metrics were explored considering responders. Significant (p < 0.05, FWE corrected) positive correlations were found between within-network RSFC of the default mode and right frontoparietal networks and most of the psychological scores (see Table 5); the lower the FC, the lower the scores related to the eating disorder symptomatology. No significant correlation was found with %EWL.
Between-network RSFC
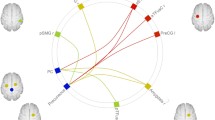
Responders showed significantly higher partial correlation values between the executive control and the salience network in respect to both controls and non-responders (p < 0.05, FWE corrected). As depicted in Fig. 3, these RSNs showed anticorrelation in responders with respect to controls and non-responders.
Between-network FC. Significant differences of between-network FC in the three groups of patients. The box plot shows the distributions of the correlation values (edge strengths): the responders showed a significantly lower correlation between the executive control and the salience networks (p < 0.05 FWE corrected, indicated with an asterisk) relative to both controls (a) and non-responders (b). On each box, the central mark indicates the median, and the bottom and top edges of the box indicate the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points not considered outliers, and the outliers are plotted individually using the “ + ” symbol
No significant differences in partial or full correlations between RSNs were found between controls and non-responders.
A significant positive correlation (Spearman rho = 0.48, p = 0.0012) was found between %EWL and executive control-salience network correlation values (edge strengths) in responders (blue) and non-responders (magenta) (Fig. 4), possibly indicating a link between these RSNs and weight loss. Significant correlations were not observed with psychological scores.
Discussion
In the present study, we explored resting-state brain functional connectivity of obese patients treated with SG, classified as responders and non-responders according to %EWL. Interestingly, we found several differences of functional connectivity in these categories of patients, possibly offering a neural correlate to the tendency of losing or regaining weight after SG, as evaluated at a minimum follow-up of 24 months after surgery.
Responders to SG demonstrated significantly lower within-network RSFC with respect to both controls and non-responders in several regions of the default mode and frontoparietal networks.
The default mode network received great attention in recent years. This network includes several key cortical regions, involved in a wide variety of high-level cognitive functions, such as self-referential processing, interoception, attention, recollection of prior experiences, and emotional processing [33, 34]: posterior cingulate cortex, precuneus, medial prefrontal cortex, and bilateral temporoparietal junction [35, 36]. Evidence on default mode network suggested that this network is never turned off, but mainly enhanced or attenuated [35, 36].
Moreover, differences between responders and non-responders were detected in regions including precuneus and posterior cingulate gyrus. Precuneus is of importance also considering the great metabolic demand, estimated about > 35% glucose consumption than any other brain region [37]. Precuneus is highly connected, especially with association regions, and it is active during episodic and autobiographical memory, suggesting a role as a hub for mediating integration of internal and external information flow [38,39,40].
The frontoparietal network is believed to be a control network, related to handle new tasks, and flexibly interact with other control or processing networks [41]. Functional abnormalities of the frontoparietal network have been suggested as a common path linking various disorders such as anxiety and depression [41].
Moreover, non-responders showed higher RSFC, with respect to controls, in regions of the lateral visual network, such as bilateral occipital poles and lateral occipital cortices. The occipital lobe is related to visual functions, and it is involved in tasks such as visuospatial processing, distance and depth estimation, color perception, shape and face recognition, and visual memory recalling [42].
Taken together, results of within-network RSFC analysis might reflect negative emotions related to memories of body image before SG in non-responders that are somehow difficult to overcome and might lead to anxiety and depression with consequent increased food consumption and inability to lose weight. This concept is further supported by the correlations that were found between default mode, right frontoparietal networks RSFC, and most of the psychological scores.
Additionally, we further investigated our sample using a between-network RSFC approach and found that responders to SG showed significantly higher anti-correlation between the executive control and the salience network with respect to controls and non-responders.
It is known that executive control and the salience network serve control functions during external goal-directed tasks and act separately according to a model of functional segregation [43,44,45]. More in-depth, salience network is involved in a broad monitoring function, perceiving salient stimuli regardless of relevance [46]; on the other hand, the executive control network is related to selective external stimuli matching task goals [47].
Results of between-network RSFC suggest that responders to SG might have differences in these networks, with respect to controls and non-responders, possibly because they managed to achieve the profoundly impactful goal of weight loss. This interpretation is reinforced by significant positive correlations observed between %EWL and executive control-salience network values in both responders and non-responders, indicative of a tight connection between these networks and the major goal of patients undergoing SG which is weight loss.
Recently, Cerit and colleagues showed statistically significant decreases in RSFC magnitude and directionality (i.e., positively correlated to anticorrelated) pre- to post-surgery within and between default mode network, salience network, and frontoparietal network nodes [48]. Here, we found similar results in responders only and not in non-responders to SG, adding novel knowledge to the current literature. However, the approach of the two studies was very different because we compared two post-surgical groups separated according to the outcome and a control group of obese patients before surgery.
Additionally, Wang et al. reported associations between regional grey matter volume and RSFC, focusing on insula and posterior cingulate cortex, suggesting a link between brain structure and function related to SG [49].
To tie up all the results of the present study and give a global interpretative message, we believe that we could be observing differences in the mental representation of body image linked to the different response to SG. Specifically, responders might have showed lower connectivity of the above-mentioned brain regions because they are less anchored to the internal representation of body image as it was before surgery. Conversely, the greater connectivity in regions involved in memory and emotion regulations of non-responders might mirror the difficult overcoming of the previous body size and shape leading to weight regain. This is in keeping with clinical observations that some of these patients tend to unjustifiably move objects (e.g., chairs or tables) to pass through tight spaces even if they could have managed to do it without moving it. Indeed, obesity is a complex and multifaceted disease. There is clear evidence that obesity is linked to poor body image, although not all obese patients show an abnormal body image perception [50]. Our hypothesis is that non-responders to SG might have a distortion of internal body image such that they continue to perceive their body as it was before surgery and not as it is after weight loss. This distortion might be based on memories leading to internalization of negative messages, possibly mirroring the observed brain fMRI changes.
The results of the present study are of broad interest because they offer novel insights into the tendency of responding or not responding to SG by closely looking at the brain functional dynamics, possibly leading to improve and develop cognitive and behavioral strategies aimed to reduce bariatric and metabolic surgery failure rate [51]. These might potentially help to reduce serious comorbidities associated to obesity [52,53,54].
Factors associated with long-term outcomes of bariatric and metabolic surgery are likely multiple and linked to each other by complex relationships. A pivotal role in weight loss and weight maintenance is played by various peptide hormones regulating appetite and glucose homeostasis. Indeed, it has been shown that fasting plasma levels of both leptin and ghrelin correlated with weight results at follow-up > 5 years after surgery, and that hormonal response to glucose during oral glucose tolerance test was different between responders and non-responders [55]. Intriguingly, leptin and ghrelin (preferentially acyl-ghrelin) are known to cross the intact blood–brain barrier and to interact with hypothalamic neural tissues such as AgRP/NPY neurons, suggesting implications for the cross-talk between peptide hormones and the brain in long-term results after bariatric surgery [56, 57]. This is further confirmed by results of Schmidt and colleagues, reporting that bariatric surgery-induced increase in ventromedial prefrontal cortex–ventral striatum RSFC, known as central hub of the brain’s reward and motivational system, was correlated with a decrease in fasting-state systemic leptin [58].
Limitations
The lack of rs-fMRI signal acquisition before surgery, which would have been important to investigate longitudinal changes of brain FC, represents a limitation of the present study. Also, a larger sample size would have been preferable; noteworthy is that the population studied is truly homogeneous as all the surgical patients were treated with SG and were followed-up for at least 2 years. Third, there were a greater number of females in our sample limiting the generalizability of results for male patients; however, this gender distribution reflected the usual distribution of patients undergoing bariatric and metabolic surgery [1,2,3,4,5,6].
Conclusion
In conclusion, the present study showed that there are differences of brain functional connectivity in patient responders or non-responders to SG.
Responders to SG showed lower within-network RSFC with respect to both controls and non-responders in the default mode and frontoparietal networks, whereas non-responders showed higher RSFC with respect to controls in lateral visual network. Moreover, responders showed significantly lower partial correlation between the executive control and the salience network with respect to controls and non-responders.
Our results add knowledge to the current literature, offer new insights into the neural correlates of outcomes in patients who undergo SG, and expand knowledge about neural mechanisms related to surgical response.
Data availability
Data are available upon reasonable requests of collaboration.
Code availability
None.
Abbreviations
- RSFC:
-
Resting-state functional connectivity
- SG:
-
Sleeve gastrectomy
- FWE:
-
Family-wise error
- EWL:
-
Excess weight loss
- fMRI:
-
Functional MRI
- BOLD:
-
Blood oxygen level-dependent
- FC:
-
Functional connectivity
- TIV:
-
Total intracranial volume
- TFCE:
-
Threshold-free cluster enhancement
- %EWL:
-
Percentage of excess weight loss
- BES:
-
Binge Eating Scale
- SOM:
-
Somatization
- OBS\COMP:
-
Obsessive-compulsive
- IS:
-
Interpersonal sensitivity
- PAR:
-
Paranoid thought
- GSI:
-
General Symptom Index
- RSNs:
-
Resting-state networks
- ICA:
-
Independent component analysis
References
Hedley AA, Ogden CL, Johnson CL, et al (2004) Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. J Am Med Assoc 291: https://doi.org/10.1001/jama.291.23.2847
Phillips BT, Shikora SA (2018) The history of metabolic and bariatric surgery: development of standards for patient safety and efficacy. Metabolism 79:97–107. https://doi.org/10.1016/j.metabol.2017.12.010
Hill JO (1979) Peters JC (1998) Environmental contributions to the obesity epidemic. Science 280:1371–1374. https://doi.org/10.1126/science.280.5368.1371
Colquitt JL, Pickett K, Loveman E, Frampton GK (2014) Surgery for weight loss in adults. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD003641.pub4
Nguyen N, Champion JK, Ponce J et al (2012) A review of unmet needs in obesity management. Obes Surg 22:956–966. https://doi.org/10.1007/s11695-012-0634-z
Golomb I, ben David M, Glass A, et al (2015) Long-term metabolic effects of laparoscopic sleeve gastrectomy. JAMA Surg 150:1051. https://doi.org/10.1001/jamasurg.2015.2202
Smith SM, Vidaurre D, Beckmann CF et al (2013) Functional connectomics from resting-state fMRI. Trends Cogn Sci 17:666–682. https://doi.org/10.1016/j.tics.2013.09.016
Mallio C, Zobel B, Quattrocchi C (2015) Evaluating rehabilitation interventions in Parkinson’s disease with functional MRI: a promising neuroprotective strategy. Neural Regen Res 10:702. https://doi.org/10.4103/1673-5374.156957
Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med 34:537–541. https://doi.org/10.1002/mrm.1910340409
Mallio CA, Piervincenzi C, Gianolio E et al (2019) Absence of dentate nucleus resting-state functional connectivity changes in nonneurological patients with gadolinium-related hyperintensity on T1-weighted images. J Magn Reson Imaging 50:445–455. https://doi.org/10.1002/jmri.26669
Quattrocchi CC, de Pandis MF, Piervincenzi C et al (2015) Acute modulation of brain connectivity in Parkinson disease after automatic mechanical peripheral stimulation: a pilot study. PLoS ONE 10:e0137977. https://doi.org/10.1371/journal.pone.0137977
Mallio CA, Piervincenzi C, Carducci F et al (2020) Within-network brain connectivity in Crohn’s disease patients with gadolinium deposition in the cerebellum. Neuroradiology 62:833–841. https://doi.org/10.1007/s00234-020-02415-x
Legget KT, Wylie KP, Cornier M-A et al (2021) Altered between-network connectivity in individuals prone to obesity. Physiol Behav 229:113242. https://doi.org/10.1016/j.physbeh.2020.113242
Moreno-Lopez L, Contreras-Rodriguez O, Soriano-Mas C et al (2016) Disrupted functional connectivity in adolescent obesity. NeuroImage Clinical 12:262–268. https://doi.org/10.1016/j.nicl.2016.07.005
Park B, Byeon K, Lee MJ et al (2020) Whole-brain functional connectivity correlates of obesity phenotypes. Hum Brain Mapp 41:4912–4924. https://doi.org/10.1002/hbm.25167
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113. https://doi.org/10.1016/0028-3932(71)90067-4
Derogatis LR, Unger R (2010) Symptom checklist-90-revised. In: The Corsini Encyclopedia of Psychology. John Wiley & Sons, Inc., Hoboken. https://doi.org/10.1002/9780470479216.corpsy0970
Fossati A, di Ceglie A, Acquarini E, Barratt ES (2001) Psychometric properties of an Italian version of the Barratt Impulsiveness Scale-11 (BIS-11) in nonclinical subjects. J Clin Psychol 57:815–828. https://doi.org/10.1002/jclp.1051
Sighinolfi C, Norcini Pala A, Chiri LR et al (2010) Difficulties in emotion regulation scale (DERS): the Italian translation and adaptation. Psicoter Cogn Comport 16:141–170
di Bernardo M, Barciulli E, Ricca V et al (1998) Binge Eating Scale in obese patients: validation of the Italian version. Minerva Psichiatr 39:125–130
Esteban O, Markiewicz CJ, Blair RW et al (2019) fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 16:111–116. https://doi.org/10.1038/s41592-018-0235-4
Gorgolewski K, Burns CD, Madison C, et al (2011) Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in Python. Frontiers in Neuroinformatics 5: https://doi.org/10.3389/fninf.2011.00013
Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005) Investigations into resting-state connectivity using independent component analysis. Philosl Trans R Soc B Biol Sci 360:1001–1013. https://doi.org/10.1098/rstb.2005.1634
Smith S, Nichols T (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. https://doi.org/10.1016/j.neuroimage.2008.03.061
Smith SM, Fox PT, Miller KL et al (2009) Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci 106:13040–13045. https://doi.org/10.1073/pnas.0905267106
Thomas Yeo BT, Krienen FM, Sepulcre J et al (2011) The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165. https://doi.org/10.1152/jn.00338.2011
Nickerson LD, Smith SM, Öngür D, Beckmann CF (2017) Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Frontiers in Neuroscience 11: https://doi.org/10.3389/fnins.2017.00115
Filippini N, MacIntosh BJ, Hough MG et al (2009) Distinct patterns of brain activity in young carriers of the APOE -ε4 allele. Proc Natl Acad Sci 106:7209–7214. https://doi.org/10.1073/pnas.0811879106
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25. https://doi.org/10.1002/hbm.1058
Hayasaka S, Nichols TE (2004) Combining voxel intensity and cluster extent with permutation test framework. Neuroimage 23:54–63. https://doi.org/10.1016/j.neuroimage.2004.04.035
Seeley WW, Menon V, Schatzberg AF et al (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. https://doi.org/10.1523/JNEUROSCI.5587-06.2007
Seeley WW (2019) The salience network: a neural system for perceiving and responding to homeostatic demands. J Neurosci 39:9878–9882. https://doi.org/10.1523/JNEUROSCI.1138-17.2019
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network. Ann N Y Acad Sci 1124:1–38. https://doi.org/10.1196/annals.1440.011
Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci 100:253–258. https://doi.org/10.1073/pnas.0135058100
Raichle ME (2015) The brain’s default mode network. Annu Rev Neurosci 38:433–447. https://doi.org/10.1146/annurev-neuro-071013-014030
Utevsky Av, Smith Dv, Huettel SA (2014) Precuneus is a functional core of the default-mode networK. J Neurosci 34:932–940. https://doi.org/10.1523/JNEUROSCI.4227-13.2014
Gusnard DA, Raichle ME (2001) Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694. https://doi.org/10.1038/35094500
Eustache F, Piolino P, Giffard B et al (2004) “In the course of time”: a PET study of the cerebral substrates of autobiographical amnesia in Alzheimer’s disease. Brain 127:1549–1560. https://doi.org/10.1093/brain/awh166
Lundstrom BN, Ingvar M, Petersson KM (2005) The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage 27:824–834. https://doi.org/10.1016/j.neuroimage.2005.05.008
Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129:564–583. https://doi.org/10.1093/brain/awl004
Marek S, Dosenbach NUF (2018) The frontoparietal network: function electrophysiology and importance of individual precision mapping. Dialogues in Clinical Neuroscience 20:133–140. https://doi.org/10.31887/DCNS.2018.20.2/smarek
Rehman A, al Khalili Y, (2021) Neuroanatomy, Occipital Lobe. StatPearls Publishing, StatPearls
Elton A, Gao W (2014) Divergent task-dependent functional connectivity of executive control and salience networks. Cortex 51:56–66. https://doi.org/10.1016/j.cortex.2013.10.012
García-García I, Jurado MÁ, Garolera M et al (2013) Alterations of the salience network in obesity: a resting-state fMRI study. Hum Brain Mapp 34:2786–2797. https://doi.org/10.1002/hbm.22104
Borowitz MA, Yokum S, Duval ER, Gearhardt AN (2020) Weight-related differences in salience, default mode, and executive function network connectivity in adolescents. Obesity 28:1438–1446. https://doi.org/10.1002/oby.22853
Weissman DH (2004) The neural mechanisms for minimizing cross-modal distraction. J Neurosci 24:10941–10949. https://doi.org/10.1523/JNEUROSCI.3669-04.2004
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215. https://doi.org/10.1038/nrn755
Cerit H, Davidson P, Hye T et al (2019) Resting-state brain connectivity predicts weight loss and cognitive control of eating behavior after vertical sleeve gastrectomy. Obesity 27:1846–1855. https://doi.org/10.1002/oby.22607
Wang Y, Ji G, Hu Y et al (2020) Laparoscopic sleeve gastrectomy induces sustained changes in gray and white matter brain volumes and resting functional connectivity in obese patients. Surg Obe Relat Dis 16:1–9. https://doi.org/10.1016/j.soard.2019.09.074
Schwartz MB, Brownell KD (2004) Obesity and body image. Body Image 1:43–56. https://doi.org/10.1016/S1740-1445(03)00007-X
Lasaponara S, Mauro F, Carducci F, et al (2017) Increased alpha band functional connectivity following the quadrato motor training: a longitudinal study. Frontiers in Human Neuroscience 11: https://doi.org/10.3389/fnhum.2017.00282
Quattrocchi CC, Giona A, di Martino A et al (2015) Lumbar subcutaneous edema and degenerative spinal disease in patients with low back pain: a retrospective MRI study. Musculoskelet Surg 99:159–163. https://doi.org/10.1007/s12306-015-0355-2
Greco F, Quarta LG, Grasso RF et al (2020) Increased visceral adipose tissue in clear cell renal cell carcinoma with and without peritumoral collateral vessels. Br J Radiol 93:20200334. https://doi.org/10.1259/bjr.20200334
Greco F, Mallio CA, Grippo R et al (2020) Increased visceral adipose tissue in male patients with non-clear cell renal cell carcinoma. Radiol Med (Torino) 125:538–543. https://doi.org/10.1007/s11547-020-01146-6
Sima E, Webb D-L, Hellström PM, Sundbom M (2019) Non-responders after gastric bypass surgery for morbid obesity: peptide hormones and glucose homeostasis. Obes Surg 29:4008–4017. https://doi.org/10.1007/s11695-019-04089-8
Cassidy RM, Tong Q (2017) Hunger and satiety gauge reward sensitivity. Frontiers in Endocrinology 8: s10.3389/fendo.2017.00104
Banks WA, Tschöp M, Robinson SM, Heiman ML (2002) Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 302:822–827. https://doi.org/10.1124/jpet.102.034827
Schmidt L, Medawar E, Aron-Wisnewsky J, et al (2021) Resting-state connectivity within the brain’s reward system predicts weight loss and correlates with leptin. Brain Communications 3: https://doi.org/10.1093/braincomms/fcab005
Funding
None.
Author information
Authors and Affiliations
Contributions
All the authors made substantial contributions to all the categories established by the International Committee of Medical Journal Editors (ICMJE) guidelines on authorship:
1. Carlo Augusto Mallio: conception and design, acquisition of data, analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
2. Giuseppe Spagnolo: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
3. Claudia Piervincenzi: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
4. Nikolaos Petsas: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
5. Danilo Boccetti: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
6. Federica Spani: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
7. Ida Francesca Gallo: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
8. Antonella Sisto: acquisition of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
9. Livia Quintiliani: acquisition of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
10. Gianfranco Di Gennaro: acquisition of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
11. Vincenzo Bruni: analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
12. Carlo Cosimo Quattrocchi: conception and design, acquisition of data, analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflicts of interest
All the authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was approved by the Ethical Committee of our institution (30/20 PAR ComEt CBM) and was designed as prospective case–control.
Consent to participate
The consent to participate was obtained from all the subjects.
Consent for publication
The consent for publication was obtained from all the subjects.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mallio, C.A., Spagnolo, G., Piervincenzi, C. et al. Brain functional connectivity differences between responders and non-responders to sleeve gastrectomy. Neuroradiology 65, 131–143 (2023). https://doi.org/10.1007/s00234-022-03043-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03043-3