Abstract
Purpose
To evaluate two advanced diffusion models, diffusion kurtosis imaging and the newly proposed mean apparent propagation factor-magnetic resonance imaging, in the grading of gliomas and the assessing of their proliferative activity.
Methods
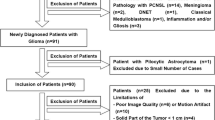
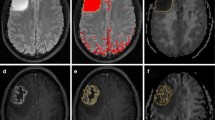
Fifty-nine patients with clinically diagnosed and pathologically proven gliomas were enrolled in this retrospective study. All patients underwent DKI and MAP-MRI scans. Manually outline the ROI of the tumour parenchyma. After delineation, the imaging parameters were extracted using only the data from within the ROI including mean diffusion kurtosis (MK), return-to-origin probability (RTOP), Q-space inverse variance (QIV) and non-Gaussian index (NG), and the differences in each parameter in the classification of glioma were compared. Receiver operating characteristic (ROC) curve analysis was used to evaluate the diagnostic performance of these parameters.
Results
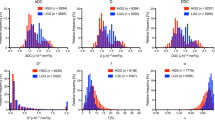
MK, NG, RTOP and QIV were significantly different amongst the different grades of glioma. MK, NG and RTOP had excellent diagnostic value in differentiating high-grade from low-grade glioma, with largest areas under the curve (AUCs; 0.929, 0.933 and 0.819, respectively; P < 0.01). MK and NG had the largest AUCs (0.912 and 0.904) when differentiating grade II tumours from III tumours (P < 0.01) and large AUCs (0.791 and 0.786) when differentiating grade III from grade IV tumours. Correlation analysis of tumour proliferation activity showed that MK, NG and QIV were strongly correlated with the Ki-67 LI (P < 0.001).
Conclusion
MK, RTOP and NG can effectively represent the microstructure of these altered tumours. Multimodal diffusion-weighted imaging is valuable for the preoperative evaluation of glioma grade and tumour proliferative activity.







Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
Code availability
DIPY (Diffusion Imaging in Python, http://nipy.org/dipy).
References
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 23(8):1231–1251. https://doi.org/10.1093/neuonc/noab106
Brat DJ, Prayson RA, Ryken TC, Olson JJ (2008) Diagnosis of malignant glioma: role of neuropathology. J Neurooncol 89(3):287–311. https://doi.org/10.1007/s11060-008-9618-1
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 15(Suppl 2):ii1-56. https://doi.org/10.1093/neuonc/not151
Reuss DE, Mamatjan Y, Schrimpf D, Capper D, Hovestadt V, Kratz A, Sahm F, Koelsche C, Korshunov A, Olar A, Hartmann C, Reijneveld JC, Wesseling P, Unterberg A, Platten M, Wick W, Herold-Mende C, Aldape K, von Deimling A (2015) IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol 129(6):867–873. https://doi.org/10.1007/s00401-015-1438-8
Richards-Taylor S, Ewings SM, Jaynes E, Tilley C, Ellis SG, Armstrong T, Pearce N, Cave J (2016) The assessment of Ki-67 as a prognostic marker in neuroendocrine tumours: a systematic review and meta-analysis. J Clin Pathol 69(7):612–618. https://doi.org/10.1136/jclinpath-2015-203340
Schoenegger K, Oberndorfer S, Wuschitz B, Struhal W, Hainfellner J, Prayer D, Heinzl H, Lahrmann H, Marosi C, Grisold W (2009) Peritumoral edema on MRI at initial diagnosis: an independent prognostic factor for glioblastoma? Eur J Neurol 16(7):874–878. https://doi.org/10.1111/j.1468-1331.2009.02613.x
Hartmann M, Jansen O, Egelhof T, Forsting M, Albert FK, Sartor K (1998) Einfluss des Hirnödems auf das Rezidivwachstum maligner Gliome [Effect of brain edema on the recurrence pattern of malignant gliomas]. Radiologe 38(11):948–53. https://doi.org/10.1007/s001170050447 (German)
Heegaard S, Sommer HM, Broholm H, Broendstrup O (1995) Proliferating cell nuclear antigen and Ki-67 immunohistochemistry of oligodendrogliomas with special reference to prognosis. Cancer 76(10):1809–13. https://doi.org/10.1002/1097-0142(19951115)76:10%3c1809::aid-cncr2820761020%3e3.0.co;2-i
Bulakbasi N, Guvenc I, Onguru O, Erdogan E, Tayfun C, Ucoz T (2004) The added value of the apparent diffusion coefficient calculation to magnetic resonance imaging in the differentiation and grading of malignant brain tumors. J Comput Assist Tomogr 28(6):735–746. https://doi.org/10.1097/00004728-200411000-00003
Marrale M, Collura G, Brai M, Toschi N, Midiri F, La Tona G, Lo Casto A, Gagliardo C (2016) Physics, techniques and review of neuroradiological applications of diffusion kurtosis imaging (DKI). Clin Neuroradiol 26(4):391–403. https://doi.org/10.1007/s00062-015-0469-9
Tan Y, Zhang H, Wang X, Qin J, Wang L, Yang G, Yan H (2019) Comparing the value of DKI and DTI in detecting isocitrate dehydrogenase genotype of astrocytomas. Clin Radiol 74(4):314–320. https://doi.org/10.1016/j.crad.2018.12.004
Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K (2005) Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53(6):1432–1440. https://doi.org/10.1002/mrm.20508
Cauter SV, Veraart J, Sijbers J, Peeters RR, Himmelreich U, Keyzer FD, Van Gool SW, Van Calenbergh F, Vleeschouwer SD, Van Hecke W, Sunaert S (2012) Gliomas: diffusion kurtosis MR imaging in grading [J]. Radiology 263(2):492–501. https://doi.org/10.1148/radiol.12110927
Özarslan E, Koay CG, Shepherd TM, Komlosh ME, İrfanoğlu MO, Pierpaoli C, Basser PJ (2013) Mean apparent propagator (MAP) MRI: a novel diffusion imaging method for mapping tissue microstructure. Neuroimage 78:16–32. https://doi.org/10.1016/j.neuroimage.2013.04.016
Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D’Arceuil H (2008) de Crespigny AJ (2008) Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage 41(4):1267–1277. https://doi.org/10.1016/j.neuroimage.2008.03.036
Avram AV, Sarlls JE, Barnett AS, Özarslan E, Thomas C, Irfanoglu MO, Hutchinson E, Pierpaoli C, Basser PJ (2016) Clinical feasibility of using mean apparent propagator (MAP) MRI to characterize brain tissue microstructure. Neuroimage 127:422–434. https://doi.org/10.1016/j.neuroimage.2015.11.027
Ma K, Zhang X, Zhang H, Yan X, Gao A, Song C, Wang S, Lian Y, Cheng J (2020) Mean apparent propagator-MRI: a new diffusion model which improves temporal lobe epilepsy lateralization. Eur J Radiol 126:108914. https://doi.org/10.1016/j.ejrad.2020.108914
Zhang J, Chen X, Chen D, Wang Z, Li S, Zhu W (2018) Grading and proliferation assessment of diffuse astrocytic tumors with monoexponential, biexponential, and stretched-exponential diffusion-weighted imaging and diffusion kurtosis imaging. Eur J Radiol 109:188–195. https://doi.org/10.1016/j.ejrad.2018.11.003
Zhao J, Wang YL, Li XB, Hu MS, Li ZH, Song YK, Wang JY, Tian YS, Liu DW, Yan X, Jiang L, Yang ZY, Chu JP (2019) Comparative analysis of the diffusion kurtosis imaging and diffusion tensor imaging in grading gliomas, predicting tumour cell proliferation and IDH-1 gene mutation status. J Neurooncol 141(1):195–203. https://doi.org/10.1007/s11060-018-03025-7
Wang X, Gao W, Li F, Shi W, Li H, Zeng Q (2019) Diffusion kurtosis imaging as an imaging biomarker for predicting prognosis of the patients with high-grade gliomas. Magn Reson Imaging 63:131–136. https://doi.org/10.1016/j.mri.2019.08.001
Takahashi S, Takahashi W, Tanaka S, Haga A, Nakamoto T, Suzuki Y, Mukasa A, Takayanagi S, Kitagawa Y, Hana T, Nejo T, Nomura M, Nakagawa K, Saito N (2019) Radiomics analysis for glioma malignancy evaluation using diffusion kurtosis and tensor imaging. Int J Radiat Oncol Biol Phys 105(4):784–791. https://doi.org/10.1016/j.ijrobp.2019.07.011
Xu J, Li H, Harkins KD, Jiang X, Xie J, Kang H, Does MD, Gore JC (2014) Mapping mean axon diameter and axonal volume fraction by MRI using temporal diffusion spectroscopy. Neuroimage 103:10–19. https://doi.org/10.1016/j.neuroimage.2014.09.006
Fick RHJ, Wassermann D, Caruyer E, Deriche R (2016) MAPL: tissue microstructure estimation using Laplacian-regularized MAP-MRI and its application to HCP data. Neuroimage 134:365–385. https://doi.org/10.1016/j.neuroimage.2016.03.046
Funding
This study has received funding by Inner Mongolia Autonomous Region Science and Technology Plan Project: Application value of multimodal functional magnetic resonance imaging in accurate evaluation of glioma (No. 2019GG047).
Author information
Authors and Affiliations
Contributions
Yang Gao and Qiong Wu contributed to the conception of the study; Peng Wang and He Zhao performed the experiment; Sheng-hui Xie and Rui Lang contributed significantly to analysis and manuscript preparation; Bo Li, Xue-ying Ma and Jin-long He performed the data analyses and wrote the manuscript; Hua-peng Zhang and Shao-yu Wang helped perform the analysis with constructive discussions.
Corresponding author
Ethics declarations
Conflict of interest
The two authors in this article (Huapeng Zhang and Shaoyu Wang) are employees of Siemens Healthcare. The remaining authors state that their products or services may not be related to any company related to the subject of the article.
Ethical approval
Institutional Review Board approval was obtained.
Informed Consent
Written informed consent was obtained from all subjects (patients) in this study.
Consent to participate
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Consent for publication
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
1.Non-Gaussian diffusion model (DKI and MAP-MRI) can better reflect the pathological state of glioma than Gaussian diffusion model.
2.High-order diffusion weighted imaging can better reflect the proliferation activity of glioma.
3.DKI and MAP-MRI are important for neurosurgeons to assess the grade of glioma before operation.
Rights and permissions
About this article
Cite this article
Xie, Sh., Lang, R., Li, B. et al. Evaluation of diffuse glioma grade and proliferation activity by different diffusion-weighted-imaging models including diffusion kurtosis imaging (DKI) and mean apparent propagator (MAP) MRI. Neuroradiology 65, 55–64 (2023). https://doi.org/10.1007/s00234-022-03000-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03000-0