Abstract
Purpose
Endoscopic biopsy is recommended for diagnosis of nasopharyngeal carcinoma (NPC). A proportion of lesions are hidden from endoscopic view but detected with magnetic resonance imaging (MRI). This systematic review and meta-analysis investigated the diagnostic performance of MRI for detection of NPC.
Methods
An electronic search of twelve databases and registries was performed. Studies were included if they compared the diagnostic accuracy of MRI to a reference standard (histopathology) in patients suspected of having NPC. The primary outcome was accuracy for detection of NPC. Random-effects models were used to pool outcomes for sensitivity, specificity, and positive and negative likelihood ratio (LR). Bias and applicability were assessed using the modified QUADAS-2 tool.
Results
Nine studies were included involving 1736 patients of whom 337 were diagnosed with NPC. MRI demonstrated a pooled sensitivity of 98.1% (95% CI 95.2–99.3%), specificity of 91.7% (95% CI 88.3–94.2%), negative LR of 0.02 (95% CI 0.01–0.05), and positive LR of 11.9 (95% CI 8.35–16.81) for detection of NPC. Most studies were performed in regions where NPC is endemic, and there was a risk of selection bias due to inclusion of retrospective studies and one case–control study. There was limited reporting of study randomization strategy.
Conclusion
This study demonstrates that MRI has a high pooled sensitivity, specificity, and negative predictive value for detection of NPC. MRI may be useful for lesion detection prior to endoscopic biopsy and aid the decision to avoid biopsy in patients with a low post-test probability of disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rationale
Nasopharyngeal carcinoma (NPC) has unique epidemiology, natural history, and treatment response necessitating distinct management paradigms [1]. It encompasses keratinizing, non-keratinizing, and basaloid type squamous cell carcinomas [2]. The non-keratinizing subtype is most common in endemic populations, including east and southeast Asia, north Africa, and the Arctic [3]. The global incidence of NPC has been increasing between 2009 and 2019, although mortality and morbidity has reduced over this period [4]. Major risk factors include genetic and family history, environmental agents such as nitrosamines in preserved foods, and exposure to formaldehyde, wood dusts, and fumes [5]. Epstein-Barr virus (EBV) infection is closely associated with NPC but its exact role in pathogenesis remains enigmatic [3, 5].
Biopsy of the primary tumor is required for definitive diagnosis [6] and is generally performed endoscopically under local or general anesthesia [7]. Although endoscopic biopsy is the recommended first step across guidelines [1, 6,7,8], some 10% of nasopharyngeal cancers are missed at initial endoscopy. This is generally attributed to small size, submucosal location [9], coexistent hyperplasia [10], and anatomic difficulty in assessing the lateral pharyngeal recess [11]. For endoscopically occult cases, current guidelines recommend repeat endoscopy and biopsy of tissue identified as abnormal on MRI or PET-CT [6].
MRI is established as the preferred modality for locoregional staging of NPC [3, 6, 7, 10, 12], due to good soft tissue visualization of parapharyngeal or masticator space involvement, perineural and intracranial spread [8, 13] with PET-CT playing a complementary role in staging of nodal and distant metastasis [14, 15]. MRI has not historically been used for primary diagnosis due to concerns about poor sensitivity for small mucosal lesions [16].
Unfortunately, the vast majority of patients present with advanced disease, with less than 8% presenting at stage I [17]. Beyond stage II, there is a substantial rate of distant metastasis [8], for which concurrent chemoradiotherapy is often indicated [3, 6, 7]. The addition of chemotherapy increases risk of acute toxicity [3]. Consequently, there has been substantial interest in screening for early NPC with EBV immunoserology, in some studies coupled with MRI [3, 18]. This has renewed interest in the role and diagnostic accuracy of MRI, with recent efforts to produce MRI diagnostic criteria [19, 20].
Objectives
The primary endpoint of this systematic review and meta-analysis is to pool the sensitivity, specificity, likelihood ratios (LRs), and hierarchical summary receiver-operating characteristics (HSROC) of MRI for primary detection of nasopharyngeal carcinoma relative to the reference standard of post-MRI endoscopic biopsy. Secondary endpoints are to identify barriers by way of qualitative or quantitative factors that influence diagnostic accuracy.
Materials and methods
Search strategy
The search strategy was devised in accordance with the revised PRISMA 2020 statement [21] and registered with PROSPERO (CRD42021252609) [22]. Twelve databases and registries were searched electronically including Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Cochrane Clinical Answers, Cochrane Methodology Register, American College of Physicians Journal Club, Database of Abstracts of Reviews of Effects, NHS Economic Evaluation Database, PubMed, PROSPERO, and Google Scholar. Search terms included (“magnetic resonance imaging (MRI)”) and (“nasopharyngeal carcinoma,” “nasopharyngeal cancer,” “nasopharynx cancer,” and “nasopharyngeal neoplasm”) as well as relevant truncations, MeSH terms, and keywords. Articles published between inception and March 2022 were included, without language restrictions. Duplicate studies were removed, and titles and abstracts were screened independently by 2 reviewers (VVG and NNN). Full texts of potentially relevant studies were obtained, and their reference lists reviewed for any additional studies.
Selection criteria
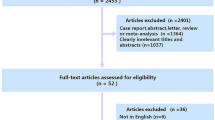
We included prospective and retrospective studies that assessed performance of MRI for diagnosis of NPC in patients with suspected disease, compared to histopathology as the reference standard. Following full-text review, articles were included if they reported sensitivity and specificity values for detection of primary NPC. Papers for which this was not possible were excluded. Articles which assessed accuracy for detection of recurrent tumor were excluded. Studies with risk of overlapping cohorts were scrutinized and the most complete dataset was included to avoid duplication of data. Abstracts, case reports, case series, editorials, and prior systematic reviews or meta-analyses were also excluded due to risk of publication bias and duplication. The selection process was summarized graphically per the PRISMA [21] guidelines using the ShinyApp tool [23] in Fig. 1.
Data extraction
Two authors (VVG and NNN) independently extracted data including study design, patient demographics, inclusion and exclusion criteria, cohort enrolment and MRI-technique (field strength, sequences), diagnostic criteria utilized, and diagnostic accuracy data (true- and false-positives and negatives, sensitivity, specificity, and positive and negative predictive values). If multiple diagnostic features were assessed, we included the parameter with best diagnostic performance in the meta-analysis. If data were ambiguous or could not be extracted into 2 × 2 tables, the authors of the paper in question were contacted for clarification where possible. Discrepancies between the 2 reviewers were resolved by discussion and consensus, and results reviewed by the senior investigator (MVC).
Methodologic quality assessment
Included studies were assessed using the revised Quality Assessment of Diagnostic Accuracy of Studies (QUADAS-2) tool [24]. This tool facilitates critical assessment of risk of bias and applicability concerns for the index and reference test for each included study, as well as methodologic assessment of study design and patient selection techniques. For each assessment, the level of risk is designated as low, high, or unclear.
Statistical analysis
Statistical analysis was performed using STATA v14.1 (StataCorp, College Station, TX). A two-tailed p-value < 0.05 was considered statistically significant. To pool diagnostic accuracy measures, we used a bivariate mixed-effects regression model that allows correlation between sensitivity and specificity. Results were presented as summary sensitivities, specificities, LRs, and ROC AUC values with 95% CIs. A positive LR > 10 or a negative LR < 0.1 was considered to be strong diagnostic evidence [25].
Interstudy variability was assumed, and an exploration of the causes of variability, including study design (prospective vs. retrospective) differences, was performed using meta-regression. Statistical analyses of variability and publication bias are not included in this report in accordance with updated recommendations by the PRISMA Diagnostic Test Accuracy Group [26]. The area under the HSROC curve was calculated, using a ≥ 0.9 threshold to indicate high test performance accuracy [27].
Results
Literature search
A total of 5886 references were identified through searches of 12 electronic databases and registers, of which 23 met criteria for full-text review. Manual search through reference lists yielded 1 additional relevant study. Following exclusion of studies with overlapping cohorts (n = 5), post hoc assessment of known nasopharyngeal tumor (n = 7), or for whom data could not be extracted into 2 × 2 tables (n = 2) or a full text could not be obtained (n = 1), there were 9 eligible studies for the meta-analysis. A summary of the study selection process according to the PRISMA format is presented in Fig. 1.
Study characteristics
All 9 included studies were single-institution studies, of which 4 were prospective [28,29,30,31] and 5 were retrospective [16, 32,33,34]. A total of 1736 patients with suspected disease underwent MRI and had histopathologic assessment, of whom 337 had a confirmed diagnosis of nasopharyngeal carcinoma. Approximately 38% of patients were female, with age ranging from 10 to 86 years.
All studies included patients suspected of having nasopharyngeal carcinoma, due to positive EBV serology, middle ear effusion, blood-stained epistaxis or rhinorrhea, cervical nodal metastases, or other similar clinical features [16, 30, 32, 33, 35]. One study included patients who presented with cervical nodal metastasis from a presumed head and neck primary source which was occult on initial clinical examination and endoscopy [34]. One study recruited from a high-risk screening cohort with positive EBV serology [28]. One case–control study included patients with proven or suspected NPC [16]; only the cohort suspected to have NPC at the time of MRI were included in our pooled analysis. All studies excluded patients for whom both imaging and histopathology were not obtained. Four studies [28,29,30, 33] excluded non-NPC primary tumors from analysis. Two studies [28, 34] excluded patients with a history of other malignancy. One study [33] excluded NPC beyond radiologic stage T1.
Two studies did not report MRI field strength or technique [32, 35]. MRI was performed at field strength of 3 T in three studies [28, 33, 34], 1.5 T in three studies [16, 29, 30], and either 1.0 or 1.5 T in one study [31]. All studies where MRI technique was listed included T1-weighted pre- and post-gadolinium spin-echo, and T2-weighted sequences with or without fat suppression. Three studies [28, 31, 34] included post-gadolinium volumetric gradient echo T1-weighted sequences. Slice thickness varied between 1 and 5 mm with intersection gaps of either 0 or 1 mm [16, 28,29,30,31, 33, 34] with two studies not reporting these variables [32, 35]. Three studies [16, 28, 30] used a multiparametric scoring system to assess likelihood of NPC, one study [33] assessed diagnostic performance of discrete imaging findings (e.g., nasopharyngeal asymmetry) and the remainder did not specify MRI diagnostic criteria.
Histopathology was used as the reference standard for NPC diagnosis in all studies. In cases where initial endoscopy was negative, repeat biopsy targeted to the imaging abnormality was used for definitive diagnosis in four studies [16, 28,29,30]. The remaining studies did not report whether biopsy was blinded to MRI results. One study [31] included surgical resection specimens in addition to endoscopic biopsy. One study included follow-up histopathology verification from a local cancer registry [28]. Absence of NPC was diagnosed by benign findings at biopsy in seven studies [29,30,31,32,33,34,35]. In the two remaining studies [16, 28], NPC was considered absent if initial endoscopy and MRI were both normal and no new abnormality was detected at clinical and imaging follow-up.
A complete summary of study details, MRI technique and patient baseline characteristics are presented in Table 1.
Methodologic quality
Potential study bias was assessed using the QUADAS-2 metric [24, 36]. Three studies were deemed at risk of selection bias due to case–control design [33], limited description of inclusion [31] or exclusion [35] criteria. Seven studies [16, 29,30,31,32,33, 35] did not clearly describe whether patients were recruited in a randomized or consecutive manner, creating an unclear risk of selection bias. In general, the spectrum of patients was representative of patients who would be investigated with endoscopic biopsy in practice. In most studies, MRI technique and interpretation criteria were well described, with interpretation blinded to endoscopic findings. Two studies [32, 35] did not describe MRI diagnostic criteria. In two studies [16, 28], patients with no lesion detected at MRI underwent follow-up imaging and clinical assessment instead of biopsy. In two studies [28, 34], endoscopy and histopathology interpretation were not blinded to MRI findings. Two studies [29, 32] did not report indeterminate or uninterpretable results and three studies [31, 32, 35] did not discuss patient withdrawals. Only one study [28] specified the time interval between MRI and endoscopic biopsy. A complete summary of the QUADAS-2 metrics is included in Table 2. There was uncertainty about applicability of selected cohort in four studies [31, 33,34,35] and regarding applicability of the index test in two studies [32, 35]. Concerns regarding applicability are summarized in Table 3.
Diagnostic accuracy of MRI
MRI assessment for nasopharyngeal carcinoma demonstrated a pooled sensitivity of 98.1% (95% CI 95.2–99.3%), specificity of 91.7% (95% CI 88.3–94.2%), negative LR of 0.02 (95% CI 0.01–0.05), and positive LR of 11.9 (95% CI 8.35–16.81). The area under the curve (AUC) of the sROC was 0.98 (95% CI 0.97–0.99) (Fig. 2).
Subgroup analysis
Study design (retrospective vs. prospective) did not demonstrate a significant impact on diagnostic sensitivity (p = 0.48) or specificity (p = 0.83) in meta-regression models. There was insufficient data regarding lesion size or tumor stage to facilitate further subgroup analysis.
Other qualitative or quantitative findings
Although MRI was generally performed prior to biopsy, only one study [28] reported the time elapsed between MRI and endoscopic biopsy (median 1.8 months). The most reported cause of false positive MRI was nasopharyngeal hyperplasia within the pharyngeal recess [16, 29, 37]. False negatives were attributed to motion degraded sequences [31] or lesions < 10 mm in size [34].
Discussion
Nasendoscopy and biopsy have long been established as the gold standard for diagnosing NPC. However, NPC may be submucosal or contained within the pharyngeal recess and thus difficult to identify at endoscopy [11], resulting in delayed diagnosis and treatment [16]. Recent meta-analyses [14, 15] have supported current practice guidelines in which MRI is employed for locoregional staging, with PET-CT playing a key role in staging of nodal and distant metastases [6, 8, 12, 38]. However, the role of MRI in primary diagnosis of NPC is yet to be established.
Our meta-analysis supports the use of MRI as an accurate primary diagnostic tool, with a pooled sensitivity of 98.1%. MRI was also found to have excellent specificity and negative LR. MRI-detected abnormalities could direct biopsy planning whereas a normal MRI examination would establish a low post-test probability of disease. The applicability of these findings is strengthened by the MRI protocols involved, which included T2-weighted and pre- and post-gadolinium T1-weighted sequences on 1, 1.5 and 3 T magnets at slice thicknesses between 1 and 5 mm, reflecting real-world heterogeneity of protocols in clinical practice [13].
The implication for current guidelines is that MRI could be the initial diagnostic test prior to endoscopic biopsy. Pre-operative MRI may increase the yield of endoscopic biopsy by alerting the surgeon to multiple lesions or lesions which may be endoscopically occult, and aid selection of patients who may benefit from intraoperative image-guided stereotactic approaches. The negative LR of MRI implies greatly reduced post-test probability of disease. MRI is a non-invasive technique which does not require anesthesia, yields excellent soft-tissue characterization of the lateral pharyngeal recess, submucosal soft tissues, and retropharyngeal nodes [13], and already has an established role in locoregional staging [6, 14, 15]. This raises the potential for use of MRI to exclude disease, reducing need for invasive biopsy.
The major pitfall for diagnosis highlighted in the included studies is false-positive results due to asymmetric nasopharyngeal hyperplasia [39], reported in up to 14% of cases [34]. Authors have attempted to distinguish this condition from NPC by assessing features such as lesion subsite, size, signal characteristics [33], and anatomic features such as the deep mucosal white line [19, 33]. Advanced imaging techniques [40] have also been investigated as potential discriminators [41,42,43]. Notably, several papers by King and colleagues [19, 20], some of which are included in this meta-analysis [16, 28, 30], have developed and applied multiparametric diagnostic criteria. Conversely, several included papers did not describe criteria for a positive test [29, 31, 32, 34, 35], limiting potential for more subgroup analysis. Lesion size is known to limit endoscopic detection sensitivity [9]. Unfortunately, there was insufficient data among the included papers to allow subgroup analysis of MRI accuracy based on lesion size or tumor stage. Further comparative studies are required for prospective validation of such grading systems.
The results of this systematic review and meta-analysis should be considered in context of several limitations. Only two studies [28, 34] reported whether patients were enrolled randomly or consecutively, with one case–control study [33] included. Combined with the inclusion of retrospective studies, and of multiple studies from a single institution, this introduces risk of selection and publication bias. Two studies [16, 28] used clinical and imaging follow-up to document absence of NPC. Although such differential verification may overestimate test accuracy, it is difficult to ethically justify biopsy in patients with no visible lesion [44]. One study [34] was deemed at high risk of diagnostic review bias [44] because endoscopy or histopathology interpretation was not blinded to MRI findings. When the initial endoscopy is negative, repeat endoscopy and biopsy targeted to imaging abnormalities is already the established standard of care [6]. Furthermore, in clinical practice, histopathologic verification of suspected malignancy is still necessary and often interpreted with awareness of prior test results, such that the requirement for blinding is not so justifiable [44]. Only one study reported time elapsed between MRI and biopsy [28], whereas the others introduce risk of ascertainment or disease progression bias [44]. Finally, the included cohort from the available literature is relatively small. Further prospective studies could assess the most robust sequences and imaging characteristics to enhance diagnostic accuracy.
Conclusion
Gadolinium-enhanced nasopharynx MRI demonstrates exceptional sensitivity, positive and negative LR for diagnosis of nasopharyngeal carcinoma with good specificity. The results support use of MRI as a primary diagnostic tool for lesion detection, in addition to its established role in locoregional staging. If used prior to endoscopic biopsy, it may increase the yield of biopsy or aid the decision to avoid biopsy in patients with low post-test probability of disease.
Data availability
Data and materials are sourced from published medical journals which have been referenced in the manuscript.
Code availability
Custom code in SAS.
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Abbreviations
- MRI:
-
Magnetic resonance imaging
- NPC:
-
Nasopharyngeal carcinoma
- CI:
-
Confidence interval
- LR:
-
Likelihood ratio
- EBV:
-
Epstein-Barr virus
- HSROC:
-
Hierarchical summary receiver-operating characteristics
- QUADAS-2:
-
Quality Assessment of Diagnostic Accuracy of Studies Version 2
References
Pastor M, Lopez Pousa A, del Barco E, Perez Segura P, Astorga BG, Castelo B, Bonfill T, Martinez Trufero J, Grau JJ, Mesia R (2018) SEOM clinical guideline in nasopharynx cancer (2017). Clin Transl Oncol 20(1):84–88
El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ (2017) WHO classification of head and neck tumours, vol 9, 4th edn. IARC Publications, Lyon
Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J (2019) Nasopharyngeal carcinoma. Lancet 394(10192):64–80
Yu H, Yin X, Mao Y, Chen M, Tang Q, Yan S (2021) The global burden of nasopharyngeal carcinoma from 2009 to 2019: an observational study based on the Global Burden of Disease Study 2019. Eur Arch Otorhinolaryngol 279:1519–1533
Tsao SW, Yip YL, Tsang CM, Pang PS, Lau VM, Zhang G, Lo KW (2014) Etiological factors of nasopharyngeal carcinoma. Oral Oncol 50(5):330–338
Bossi P, Chan AT, Licitra L, Trama A, Orlandi E, Hui EP, Halamkova J, Mattheis S, Baujat B, Hardillo J et al (2021) Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 32(4):452–465
Simo R, Robinson M, Lei M, Sibtain A, Hickey S (2016) Nasopharyngeal carcinoma: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol 130(S2):S97–S103
Saba NF, Salama JK, Beitler JJ, Busse PM, Cooper JS, Jones CU, Koyfman S, Quon H, Ridge JA, Siddiqui F et al (2016) ACR appropriateness criteria(R) for nasopharyngeal carcinoma. Head Neck 38(7):979–986
Sham JST, Choy D, Wei WI, Ng MH, Zong Y-S, Guo Y-Q, Luo Y (1990) Detection of subclinical nasopharyngeal carcinoma by fibreoptic endoscopy and multiple biopsy. Lancet 335(8686):371–374
King AD (2022) MR imaging of nasopharyngeal carcinoma. Magn Reson Imaging Clin N Am 30(1):19–33
Loh LE, Chee TSG, John AB (1991) The anatomy of the fossa of Rosenmuller - its possible influence on the detection of occult nasopharyngeal carcinoma. Singapore Med J 32:154–155
Tang LL, Chen YP, Chen CB, Chen MY, Chen NY, Chen XZ, Du XJ, Fang WF, Feng M, Gao J et al (2021) The Chinese Society of Clinical Oncology (CSCO) clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma. Cancer Commun (Lond) 41(11):1195–1227
Olliff J, Richards P, Connor S, Wong WL, Beale T, Madani G (2014) Head and neck cancers. Recommendations for cross-sectional imaging in cancer management. The Royal College of Radiologists, London
Vellayappan BA, Soon YY, Earnest A, Zhang Q, Koh WY, Tham IW, Lee KM (2014) Accuracy of (18)F-flurodeoxyglucose-positron emission tomography/computed tomography in the staging of newly diagnosed nasopharyngeal carcinoma: a systematic review and meta-analysis. Radiol 48(4):331–338
Chen WS, Li JJ, Hong L, Xing ZB, Wang F, Li CQ (2016) Comparison of MRI, CT and 18F-FDG PET/CT in the diagnosis of local and metastatic of nasopharyngeal carcinomas: an updated meta analysis of clinical studies. Am J Translat Res 8(11):4532–4547
King AD, Vlantis AC, Tsang RKY, Gary TMK, Au AKY, Chan CY, Kok SY, Kwok WT, Lui HK, Ahuja AT (2006) Magnetic resonance imaging for the detection of nasopharyngeal carcinoma. Am J Neuroradiol 27(6):1288–1291
Jen C-W, Tsai Y-C, Wu J-S, Chen P-L, Yen J-H, Chuang W-K (2020) Cheng SH-C: Prognostic classification for patients with nasopharyngeal carcinoma based on American Joint Committee on cancer staging system T and N categories. Ther Radiol Oncol 4:2–2
Chan KCA, Woo JKS, King A, Zee BCY, Lam WKJ, Chan SL, Chu SWI, Mak C, Tse IOL, Leung SYM et al (2017) Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med 377(6):513–522
King AD, Woo JKS, Ai QY, Mo FKF, So TY, Lam WKJ, Tse IOL, Vlantis AC, Yip KWN, Hui EP et al (2020) Early detection of cancer: evaluation of MR imaging grading systems in patients with suspected nasopharyngeal carcinoma. Am J Neuroradiol 41(3):515–521
King AD, Wong LYS, Law BKH, Bhatia KS, Woo JKS, Ai QY, Tan TY, Goh J, Chuah KL, Mo FKF et al (2018) MR imaging criteria for the detection of nasopharyngeal carcinoma: discrimination of early-stage primary tumors from benign hyperplasia. Am J Neuroradiol 39(3):515–523
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Systematic review: what is the accuracy of MRI in primary diagnosis of nasopharyngeal carcinoma? https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021252609. Accessed 29 Apr 2022
Haddaway NR, Page MJ, Pritchard CC, McGuinness LA (2022) PRISMA2020: an R package and shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Zenodo. https://doi.org/10.1002/cl2.1230
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM (2011) Group Q-: QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155(8):529–536
Deeks JJ, Altman DG (2004) Diagnostic tests 4: likelihood ratios. BMJ 329(7458):168–169
McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, and the P-DTAG, Clifford T, Cohen JF, Deeks JJ, Gatsonis C et al (2018) Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA 319(4):388–396
Swets JA (1988) Measuring the accuracy of diagnostic systems. Science 240(4857):1285–1293
Liu Z, Li H, Yu KJ, Xie SH, King AD, Ai QH, Chen WJ, Chen XX, Lu ZJ, Tang LQ et al (2021) Comparison of new magnetic resonance imaging grading system with conventional endoscopy for the early detection of nasopharyngeal carcinoma. Cancer 127(18):3403–3412
Gao Y, Zhu SY, Dai Y, Lu BF, Lu L (2014) Diagnostic accuracy of sonography versus magnetic resonance imaging for primary nasopharyngeal carcinoma. J Ultrasound Med 33(5):827–834
King AD, Vlantis AC, Bhatia KSS, Zee BCY, Woo JKS, Tse GMK, Chan ATC, Ahuja AT (2011) Primary nasopharyngeal carcinoma: diagnostic accuracy of MR imaging versus that of endoscopy and endoscopic biopsy. Radiology 258(2):531–537
Held P, Breit A (1994) MRI and CT of tumors of the pharynx: comparison of the two imaging procedures including fast and ultrafast MR sequences. Eur J Radiol 18(2):81–91
Shayah A, Wickstone L, Kershaw E, Agada F (2019) The role of cross-sectional imaging in suspected nasopharyngeal carcinoma. Ann R Coll Surg Engl 101(5):325–327
Wang ML, Wei XE, Yu MM, Li WB (2017) Value of contrast-enhanced MRI in the differentiation between nasopharyngeal lymphoid hyperplasia and T1 stage nasopharyngeal carcinoma. Radiol Med 122(10):743–751
Yoo MG, Kim J, Bae S, Ahn SS, Ahn SJ, Koh YW (2018) Detection of clinically occult primary tumours in patients with cervical metastases of unknown primary tumours: comparison of three-dimensional THRIVE MRI, two-dimensional spin-echo MRI, and contrast-enhanced CT. Clin Radiol 73(4):e9-410
Bercin S, Yalciner G, Muderris T, Gul F, Deger HM, Kiris M (2017) Pathologic evaluation of routine nasopharynx punch biopsy in the adult population: is it really necessary? Clin 10(3):283–287
Yang B, Mallett S, Takwoingi Y, Davenport CF, Hyde CJ, Whiting PF, Deeks JJ, Leeflang MMG, Groupdagger Q-C, Bossuyt PMM et al (2021) QUADAS-C: A tool for assessing risk of bias in comparative diagnostic accuracy studies. Ann Intern Med 174(11):1592–1599
King AD, Woo JKS, Ai QY, Chan JSM, Lam WKJ, Tse IOL, Bhatia KS, Zee BCY, Hui EP, Ma BBY et al (2019) Complementary roles of MRI and endoscopic examination in the early detection of nasopharyngeal carcinoma. Ann Oncol 30(6):977–982
Gairola M, Prabhash K, Babu G, Chaturvedi P, Kuriakose M, Birur P, Anand A, Kaushal A, Mahajan A, Syiemlieh J et al (2020) Indian clinical practice consensus guidelines for the management of nasopharyngeal cancer. Indian Journal of Cancer 57(5 Supplement 1):S9–S11
Nordin AJ, Secondino S, Rahim NA, Pedrazzoli P, Siena S, Rossetti C, Aris T (2009) Imaging in nasopharyngeal carcinoma: the value of 18-florine fluorodeoxyglucose PET/CT in comparison to conventional imaging modalities CT and MRI. Radiol Oncol 43(4):247–257
Ai QYH, Chen W, So TY, Lam WKJ, Jiang B, Poon DMC, Qamar S, Mo FKF, Blu T, Chan Q et al (2020) Quantitative T1rho MRI of the head and neck discriminates carcinoma and benign hyperplasia in the nasopharynx. Am J Neuroradiol 41(12):2339–2344
Ai QY, King AD, Chan JSM, Chen W, Chan KCA, Woo JKS, Zee BCY, Chan ATC, Poon DMC, Ma BBY et al (2019) Distinguishing early-stage nasopharyngeal carcinoma from benign hyperplasia using intravoxel incoherent motion diffusion-weighted MRI. Eur Radiol 29(10):5627–5634
Yu XP, Hou J, Li FP, Wang H, Hu PS, Bi F, Wang W (2016) Intravoxel incoherent motion diffusion weighted magnetic resonance imaging for differentiation between nasopharyngeal carcinoma and lymphoma at the primary site. J Comput Assist Tomogr 40(3):413–418
Zhang SX, Jia QJ, Zhang ZP, Liang CH, Qiu QH, Chen WB, Guo MY (2013) Intravoxel incoherent motion diffusion-weighted imaging in differential diagnosis of primary nasopharyngeal carcinoma and nasopharyngeal hyperplasia. Chin J Radiol (China) 47(7):617–621
Reitsma JB, Rutjes AWS, Whiting P, Vlassov VV, Leeflang MMG, Deeks JJ (2009) Chapter 9: assessing methodological quality. In: Deeks JJ, Bossuyt PM, Gatsonis C (eds) Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy, vol 2021, Version 1.0.0. The Cochrane Collaboration. http://srdta.cochrane.org/. Accessed 29 Apr 2022
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
See separate attached document “Author Contribution List” as requested per submission guidelines.
Corresponding author
Ethics declarations
Competing interests
No conflicts of interest or competing interests.
Ethics approval
Not applicable as this is a systematic review per PRISMA Guidelines.
Consent to participate
Not relevant, as no individual patient data was used.
Consent for publication
All authors consent to publication and this study has not been accepted for publication elsewhere.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gorolay, V., Niles, N., Huo, Y. et al. MRI detection of suspected nasopharyngeal carcinoma: a systematic review and meta-analysis. Neuroradiology 64, 1471–1481 (2022). https://doi.org/10.1007/s00234-022-02941-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-02941-w