Abstract
Purpose
Vascular complications can be seen in various viral CNS infections. Variable neuro-imaging findings have been described in the literature elucidating the parenchymal changes with vascular involvement. Vessel wall imaging (VWI) can help to detect these vascular involvements. We aimed to describe the role and usefulness of VWI in the evaluation of various viral CNS infections.
Methods
In this prospective study, we included 15 cases of various diagnosed viral CNS infections (varicella, HIV encephalopathy, HSV encephalitis, Japanese encephalitis, dengue, COVID-19). VWI and time-of-flight MR angiography (TOF MRA) were included in imaging protocol. All cases were evaluated for the presence of cerebral parenchymal changes, vascular enhancement, and vascular stenosis.
Results
We found infarctions in all 5 cases of varicella, 1 case of HIV encephalopathy, and 1 case of COVID-19 encephalopathy. All these cases also showed vascular enhancement and stenosis on VWI. The rest of the cases, including 1 case of HIV encephalopathy, 3 cases of herpes encephalitis, 2 cases of dengue, and 2 cases of Japanese encephalitis did not have any vascular complication, and also did not show vascular enhancement or stenosis.
Conclusion
VWI can be useful in the detection of vascular involvement in various viral infections of CNS which show a relatively higher cerebrovascular complication rate like varicella, HIV encephalopathy, and COVID-19. However, VWI may not be useful in the routine evaluation of other viral infections like herpes, dengue, and Japanese encephalitis, which have a very low rate of cerebrovascular complication rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secondary infectious vasculitis is a morbid complication of viral infections and is known to occur in HIV, HSV, CMV, varicella, HCV, HBV, dengue, enterovirus, coxsackievirus, and COVID-19 infections. [1,2,3,4,5]. It leads to complications like ischemic stroke or intracranial hemorrhages which worsens the prognosis. Therefore, early detection of vascular involvement is of utmost importance as it can guide to initiate early treatment in the form of anti-inflammatory and anti-platelets to prevent the occurrence of complications. The clinical manifestations of these vascular complications overlap symptomatology of viral encephalitis, and can be missed. Therefore, imaging plays a vital role to detect vascular involvement. However, routine imaging modalities like MR angiography, CT angiography, and digital subtraction angiography give information about luminal involvement only, in the form of stenosis, beading, or irregularity. High-resolution vessel wall imaging is a very promising tool to directly assess the pathology of vascular walls by suppressing CSF and luminal blood, which makes vessel wall conspicuous and thus can be of great help to diagnose secondary viral vasculitis [6]. The wall thickness of intracranial vessels is in the range of 0.2–0.3 mm. However, this thickness is still smaller than the voxel size of currently available scanning softwares; but vessel wall can be visualized by adequately suppressing blood and CSF within the voxel by various methods. Higher magnetic field (3 T over 1.5 T) is preferred for VWI because of better signal-to-noise ratio. Images can be acquired in either 2D or 3D mode. Advantages of 3D scan are high signal-to-noise ratio, isotropic voxels, multiplanar images, better suppression of arterial signal, and low specific absorption rate. Isotropic voxels help to better visualize the pathology in multiple planes. On the other hand, advantages of 2D imaging are higher in-plane spatial resolution, sharper images due to reduced signal decay, and less susceptibility to motion artefacts. Disadvantage of 2D scanning is partial volume effects due to its anisotropic nature.
The various proposed mechanisms of vasculitis in viral infections include endothelial dysfunction, through vasa vasorum, immune-mediated mechanisms, transaxonal spread, or coagulopathic effects [7,8,9]. There may be resultant thickening of the vessel wall to various degrees causing vascular stenosis or occlusions leading to infarctions. The vessels also become friable and prone to hemorrhages and aneurysm formation.
Various studies have shown detection of vasculitic changes by VWI in various infections of CNS, including viral [10,11,12]. Cheng-Ching et al. have shown that VWI not only helps in diagnosis of varicella vasculopathy, but also has an immense role in guiding treatment duration and assessing treatment response [11]. Role of VWI in diagnosing vasculopathy in COVID-19, herpes encephalitis, HIV encephalopathy, and HCV encephalopathy have also been described in literature [5, 13,14,15]. In this study, we aim to assess vascular complications of various viral CNS infections using high-resolution VWI, which may help to better understand the pathophysiology of secondary viral vasculitis and can have impact on management protocol.
Materials and methods
All consecutive cases of suspected viral encephalitis (n = 26) over a period of 1 year, referred for routine MRI evaluation underwent VWI. The cases were followed up for final serological/CSF diagnosis. Only those cases, in which final diagnosis could be made were included in the study (n = 16). One case was excluded due to poor quality images due to severe motion artefacts. Finally, 15 cases were included in the study. These were 5 cases of varicella, 2 cases of HIV encephalopathy, 3 cases of HSV encephalitis, 2 cases of Japanese encephalitis, 2 cases of dengue encephalitis, and 1 case of COVID-19 encephalopathy. VWI and TOF MRA were incorporated in the routine imaging protocol. All cases of varicella vasculopathy had a prior history of varicella infection with CSF pleocytosis and positive anti-VZV antibodies in CSF. Two cases of HIV encephalopathy had CD4 count of 255 and 115 respectively. All three cases of HSV encephalitis had positive CSF PCR for HSV. Cases of Japanese encephalitis and dengue had positive CSF antibodies. The case of COVID-19 encephalopathy had positive nasal swab RT-PCR, but negative CSF RT-PCR.
Vessel wall imaging MRI acquisition
All cases underwent MR scanning in either 3 T Philips Ingenia Koninklijke MRI System, Best, Netherlands or 1.5 T Siemens Magnetom Aera System, Erlangen, Germany. For vessel wall imaging, high-resolution pre- and post-contrast 3D T1 FS sequences (VISTA and SPACE in Philips and Siemens respectively) were acquired. MRI protocol on 3 T Philips scanner were TR = 400 ms, TE = 19 ms, flip angle = 90, FOV = 202, slice thickness = 1, matrix = 240, and number of slices = 200. Protocol on 1.5 Siemens scanner were TR = 600 ms, TE = 7.2 ms, flip angle = 90, FOV = 250, slice thickness = 1, matrix = 256, and number of slices = 192. Other routine sequences, i.e., T1WI, T2WI, FLAIR, SWI, DWI, and post-contrast T1 MPRAGE and additional 3D TOF MRA were also acquired.
Image analysis
Evaluations of supraclinoid ICA, ACA, MCA, PCA, and basilar artery were done up to second-order branches for the presence of enhancement and pattern of enhancement (either smooth or nodular). Stenosis of the arteries was measured on VWI and TOF MRA and were classified into grade I (< 50% stenosis), grade II (50–70% stenosis), and grade III (> 70% stenosis). Analysis VWI and TOF MRA was done by two experienced radiologists (SV and NC) independently who were blinded from clinical details and other imaging findings.
Statistical analysis
Interobserver agreement for findings of VWI and TOF MRA were assessed using Cohen’s kappa statistics. Those results which were not in agreement were re-evaluated and result with consensus opinion was reached. Chi-square test/Fisher exact test were used to assess association of infarct with vessel wall enhancement. All statistical analysis was made using Statistical Package for Social Sciences (SSPS) software.
Results
The age, sex, diagnosis, and vascular imaging details of all cases are shown in Table 1. Age range was 1–53 years. Mean age was 15.9 (± 15.4) years. Sixty percentage of patients were male and 40% were female. There was substantial to perfect interobserver agreement for vessel wall enhancement on VWI and degree of stenosis on VWI and TOF MRA. Cohen’s kappa value ranged from 0.73 to 1 for different arteries. Infarcts were seen in 7 cases (46.7%) which included all 5 cases of varicella, 1 case of HIV encephalopathy, and 1 case of COVID-19 encephalopathy (Table 1). Acute infarcts were seen in 5 cases (3 cases of varicella, 1 case of HIV encephalopathy, and a case of COVID-19 encephalopathy). Chronic infarcts were seen in 2 cases of varicella vasculopathy. All these cases with infarcts also showed vessel wall enhancement. None of the cases without infarct showed enhancement on VWI. Eight cases without infarct did not show any vessel wall enhancement. There was significant association of infarction and vessel wall enhancement (p = 0.0002). Single territory infarcts were seen in two cases of varicella and case of COVID-19 encephalopathy, while multi-territory infarcts were seen in 3 cases of varicella and a case of HIV encephalopathy. Infarcts of varicella vasculopathy were localized to anterior circulation (MCA territory) in 2 cases, posterior circulation in 2 cases, and mixed anterior as well as posterior circulation in 1 case (Figs. 1 and 2). MCA and basilar artery were common sites of enhancement in varicella vasculopathy. Multiple sites of arterial enhancement were seen in HIV encephalopathy and COVID-19 encephalopathy (Fig. 3). The enhancement pattern was smooth in all cases. The infarcts were seen in the territory of involved vessels in all cases. All cases showing vessel wall enhancement also showed moderate to severe stenosis. Similar findings were appreciated on TOF MRA. Out of all 7 cases showing infarction, 5 cases showed moderate to severe stenosis of the responsible artery and 2 cases showed arterial occlusion on TOF MRA.
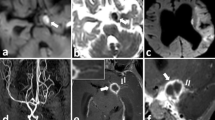
a, b, c A case of focal cerebral arteriopathy in a 1-year-old female presenting with right hemiparesis with a history of varicella infection 15 days back. a DWI shows patchy restricted diffusion in left MCA territory suggestive of subacute infarction. b MIP axial TOF MRA image shows severe focal stenosis of left M1 MCA (arrow). c Post-contrast coronal VWI shows focal smooth circumferential enhancement in left M1 MCA with severe stenosis (arrow). d, e, f Another case of varicella vasculitis in a 7-year-old female with a history of varicella infection 10 days prior presented with altered sensorium and left hemiparesis. d DWI shows acute infarcts in bilateral cerebellum and pons. e Maximum intensity projection coronal TOF MRA shows near-total occlusion of the proximal half of basilar artery (arrow) with attenuation of the distal basilar artery and bilateral distal V4 vertebral arteries. f Axial post-contrast VWI shows circumferential enhancement of proximal basilar artery with moderate to severe stenosis (arrow)
a, b, c A 2-year-old male with history of left hemiparesis since 1 month and prior varicella infection. a T2 axial image shows subacute to chronic infarction involving right cerebral hemisphere. b MIP coronal TOF MRA image shows severe attenuation of right ICA from origin till supraclinoid segment (arrow). Right M1 and M2 MCA also show moderate stenosis (arrowhead). c Post-contrast axial VWI shows focal smooth circumferential enhancement of right cavernous ICA with severe stenosis (arrow). d, e, f Another case of varicella vasculitis in a 7-year-old male with a history of posterior circulation stroke 1.5 months back with preceding history of varicella infection. d T2 axial image shows chronic infarct in pons (arrow). Another chronic infarct was seen in left PCA territory (not shown). e Maximum intensity projection coronal TOF MRA shows near-total occlusion of the distal half of basilar artery (arrow) and attenuation of bilateral distal V4 vertebral arteries. f Axial post-contrast VWI shows circumferential enhancement of basilar artery with moderate to severe stenosis (arrow)
a, b, c A 53-year-old HIV seropositive patient presented with headache and right hemiparesis. a DWI shows acute infarcts in bilateral basal ganglia, left posterior limb of the internal capsule, and bilateral thalami. b Axial MIP TOF MRA image shows severe stenosis of bilateral M1 MCA (arrows) and left P1 PCA (arrowhead). c Axial post-contrast VWI shows smooth enhancement at right MCA bifurcation (arrow). Mild enhancement can also be appreciated in bilateral P1 PCA (arrowheads). Enhancement of bilateral A1 ACA and left MCA was also present (not shown). d, e, f An 11-year-old COVID-19 positive boy presented with acute onset right hemiparesis and aphasia. d DWI showed acute infarct in the left MCA territory. e Axial MIP TOF MRA image showed focal stenosis in left M2 MCA (arrow). f Oblique sagittal reconstruction of post-contrast VWI shows circumferential enhancement in left M2 MCA with mild to moderate stenosis (arrow). Circumferential enhancement was also seen in left communicating ICA, proximal basilar artery and left V4 VA (not shown)
The rest of the cases, including one case of HIV encephalopathy, 3 cases of herpes encephalitis, 2 cases of dengue, and 2 cases of Japanese encephalitis did not have any infarcts, and also did not show enhancement of arterial walls or any stenosis. SWI showed petechial hemorrhages in infarcts of two cases of varicella vasculopathy. Microhemorrhages were also seen in one case of dengue encephalitis and two cases of herpes encephalitis.
Discussion
Varicella
Varicella zoster virus (human herpesvirus 3) is a DNA virus. It spreads through aerosols and contact with secretions from the vesicular rashes. The virus remains dormant in the ganglion of the nerves. Immunocompromised status causes reactivation of the virus and also increases the risk of vasculopathy. The reactivated viruses which were dormant in the trigeminal nerve spread transaxonally and can involve the branches of major blood vessels nearby. Other manifestations of reactivation are post-herpetic neuralgia, herpes zoster, and retinal necrosis, etc. Varicella vasculitis is the most common viral vasculitis. Post varicella vasculopathy is defined as stroke occurring within 1 year of varicella infection. It constitutes 7–31% of cases of acute ischemic stroke of childhood [16]. Varicella is responsible for transient cerebral arteriopathy in 40% of cases [17]. Infarcts can affect the cortex, superficial or deep white matter, but predominantly, it causes lesions at gray-white junction [1]. The angiographic imaging features are the same as other forms of vasculitis, i.e., segmental narrowing, thrombosis, and beading. [18]. In a series of 30 patients of VZV vasculopathy, 70% of cases had shown presence of vascular abnormality on imaging [19]. All five cases of varicella vasculopathy in our study sample had territorial infarction. We found vessel wall enhancement and narrowing of MCA in 3 cases, near-total occlusion and enhancement of basilar artery in the two cases, and near-total occlusion and enhancement of ICA in one case (Fig. 1, 2). Chen Ching et al. studied 6 patients of varicella vasculopathy with vessel wall imaging and reported enhancement of proximal large intracranial vessels, i.e., ICA, proximal M1 MCA, proximal A1 ACA, and proximal P1 PCA in 5 patients [11]. Tsivgoulis et al. had reported a case of varicella vasculitis in a 21-year-old female with hemiparesis, showing strong enhancement and severe stenosis of left terminal ICA on VWI [20].
Large vessels are commonly involved in immunocompetent patients, while small vessels are involved in immunocompromised patients. Nagel et al. had reported involvement of large arteries in 13% of patients, small arteries in 37%, and both small and large arteries in 50% of cases [19]. Imaging studies may be normal when only distal small arteries are involved. All our cases had shown the involvement of medium and larger arteries only, and enhancement was well appreciated on VWI.
Involvement of terminal ICA and proximal MCA is common and appearance can mimic Moya-Moya disease [21]. Multifocal areas may be involved. Arterial dissections, fusiform aneurysms, and dolichoectasia may also be seen. Superficial temporal arteries (STA) are also a common site of involvement, mimicking giant cell arteritis [22]. The differentiation is important as the treatment of both conditions are different. Steroids are used in giant cell arteritis while it is contraindicated in VZV vasculitis. The presence of other findings like fusiform aneurysms etc. in VZV vasculitis may help in differentiation. None of our cases had Moya-Moya pattern or STA involvement.
VWI can be used to determine the duration of treatment and to evaluate the effectiveness of treatment administered which is reflected by reduction in the extent of enhancement on VWI. This is especially important in varicella vasculopathy, where enhancement may continue up to 21 months and thus guides treatment duration [11]. VWI also helps in localizing the segment of the vessel which shows the most prominent disease process, which helps in taking a biopsy from the involved vessel for confirmatory diagnosis.
The sensitivity of TOF MRA in the detection of varicella vasculopathy is less than 70% and specificity is even lower. VWI, on the other hand, can accurately depict the mural pathology comparable with histological examinations [19]. However, in our study, all cases with vascular enhancement on VWI also showed stenosis on TOF MRA.
HIV
HIV is a common cause of viral vasculopathy and is more commonly seen in children. However, ischemic events per se in HIV are not common and range from 9 to 12% [23]. Severe immune suppression; neonatal infection; and presence of other conditions, like lymphoma, other opportunistic infections, and hypercoagulability predispose to the development of infarction. Direct viral infiltration and immune-mediated mechanisms have been proposed to explain the etiopathogenesis of HIV vasculopathy. Small, medium, or large, any vessel may be involved. Histopathological studies have demonstrated hyaline thickening of small vessels, periarteritis, and dilated VR spaces. Vascular complications may be seen with CD4 count below 400 24. Monoclonal anti-gp41 antibody against the arterial wall also leads to the development of fusiform aneurysms. Atherosclerotic disease can develop prematurely as an adverse effect of protease inhibitors. With antiretroviral treatment (HAART), the morbidity and mortality of HIV is reducing; however, with increased longevity of patients, the incidence of vascular complications is increasing. Cape town registry has reported clinical evidence of HIV vasculopathy in 20% of HIV-positive individuals [25].
We had three HIV-positive cases, among which one case was a 53-year-old female, with CD4 count of 255, who had acute infarcts in bilateral basal ganglia and thalami. Multiple vessels showed stenosis and enhancement on VWI (Fig. 3a–c). Similar to our case, Arktout et al. reported a case of HIV vasculopathy with bilateral basal ganglia infarcts and vessel wall imaging revealed multifocal thickening and circumferential enhancement of bilateral M1 MCA [26]. Cheron et al. reported a case of HIV vasculopathy with CD4 count of 313 and right lower limb recurrent weakness, which showed circumferential thickening and enhancement of left ACA and distal branches of right MCA [14]. On administration of antiretroviral therapy, the vasculitic features resolved, which suggested direct involvement of the arterial wall by the virus.
HSV
HSV is also known to be associated with ischemic infarcts. Hauer et al. studied 38 patients of herpes simplex encephalitis and found ischemic stroke in 10 patients, ICH in 27 patients, and cerebral sinovenous thrombosis in 1 patient. Vasculitis was seen in 63% of cases, predominantly in large vessels [8]. Fan et al. reported cerebrovascular complications in 17% of HSV encephalitis cases and enhancement on VWI was seen in 8.3% of cases [15]. We had three cases of herpes encephalitis, none of which showed any infarction or vasculitic features.
JE
Japanese encephalitis is the most common cause of endemic encephalitis, spread by Culex mosquitoes. Pigs and heron-like birds act as intermediate hosts. Japanese encephalitis may present with hemiplegia mimicking stroke; however, vasculopathy secondary to JE has not been reported in the literature in our knowledge. We had two patients of JE, none of them showed any infarction or vasculitic features.
Dengue
Ischemic stroke in dengue is rare and has been reported in 0.26% of cases [27]. Dengue is also reported to cause immune-mediated vasculitis. One such case has been reported in literature where vasculitic features in multiple intracranial arteries were seen with multiple infarcts in anterior and posterior circulation [28]. None of our two cases of dengue encephalopathy showed any features of vasculitis or infarcts.
SARS- CoV-2
In this pandemic era of COVID-19, ischemic stroke in COVID-positive patients is not uncommon. It causes vascular occlusion and strokes via cumulative effects of endothelial dysfunction, cytokine storm, increased procoagulant, and pro-inflammatory factors [2]. We also had one case of stroke in COVID-19 infection in an 11-year-old boy, who presented with fever, rashes, right hemiparesis, and aphasia. Acute infarct in left MCA territory was seen and TOF MRA showed focal moderate stenosis in left M2 MCA. VWI showed segments of circumferential enhancement and grade 2 stenosis in left M2 and M3 MCA (Fig. 3d–f). Enhancements were also seen in left communicating ICA, proximal basilar artery, and left distal V4 VA, suggesting a multifocal or diffuse pattern of vasculopathy. VWI has been reported to detect vascular involvement in around 85% of cases of COVID-19 encephalopathy [13]. However, no significant correlation between infarction or hemorrhage with vessel wall enhancement was found.
Other viral infections which also have been reported to cause secondary vasculitis, include hepatitis C, hepatitis B, enterovirus, coxsackievirus, hantavirus, nipah virus, influenza A, and ebolavirus [9]. Caldas et al. had reported a case of HCV mediated vasculitis who presented with acute infarct in ACA territory and MRI revealed the involvement of multiple vessels with stenosis and enhancement [5]. Hepatitis B is known to be associated with polyarteritis nodosa [29]. It affects medium to large vessels and can cause vascular occlusion, thrombosis, and aneurysms.
The enhancement pattern in all our positive cases was smooth and circumferential. Smooth enhancement implies diffuse involvement of the vessel wall secondary to leukocytic infiltration and contiguous extension from inflamed meninges [30]. On the other hand, nodular enhancement implies that there is focal pathology in the arterial wall, like development of granuloma or chronic proliferative angiitis [10, 31]. Previous studies have shown nodular or eccentric enhancement predominates in tubercular infections, which incites chronic granulomatous inflammation [10]. However, further studies with histopathological correlation are needed in this regard.
For, vessel wall imaging, both pre and post-contrast studies are needed for better assessment of enhancement. Vessel wall imaging performed without contrast can show wall thickening and degree of stenosis can also be assessed, which may be helpful in patients with deranged renal function. However, for definite detection of vascular involvement, both pre and post-contrast vessel wall imaging is recommended. No additional contrast is needed for the study. Scanning can be done in same sitting as for routine contrast-enhanced MRI of brain. In all our cases, both non-contrast and contrast-enhanced VWI was performed.
Imaging was done in acute as well as chronic phase of disease for different patients. In 12 cases, imaging was done in acute phase, i.e., within 10 days of symptom onset, 5 of whom showed acute infarction and vessel wall enhancement. In 3 cases, imaging was done in chronic phase, i.e., 1–1.5 months later, out of which, 2 showed chronic infarctions with vessel wall enhancement. Chronic persistence of vessel wall enhancement is known in varicella vasculopathy and primary CNS angiitis [11, 32].
Early detection of vessel wall involvement may have impact on treatment protocols. Anti-platelets and anti-inflammatory drugs can be started if vascular involvement is suspected, in order to prevent catastrophic vascular complications, like infarction and hemorrhage. Misra et al. had previously described role of aspirin in prevention of stroke in tubercular meningitis [3]. Moreover, VWI can guide treatment duration, as persistent vascular enhancement indicates active disease especially in cases of varicella vasculopathy. Also, VWI should be included in follow-up imaging in order to assess treatment response in terms of resolution or persistence of vascular enhancement.
Our study suffers from the limitation of a small study sample. Large-scale studies are warranted to prove the role of vessel wall imaging in these viral infections. Although all our cases were confirmed viral infections, we did not have any histopathological correlation of the vasculopathy. We did not include distal vessel assessment in our study as it is difficult to evaluate distal small branches on VWI. So, there is a possibility that distal vascular involvement might be present but were not evaluated. Imaging was done on 3 T as well as 1.5 T MRI scanner. We found better subjective resolution of images on 3 T and there is a possibility that imaging findings could have been underassessed on 1.5 T scanner. However, we did not perform any statistical analysis for differences in the quality and imaging findings on 3 T versus 1.5 T.
Conclusion
Cerebrovascular complications are seen in various viral CNS infections. VWI plays a very important role to detect vascular involvement in these infections and helps to understand the underlying pathophysiological mechanisms. Early treatment can be started on detection of vascular involvement to prevent complications and can have impact on management protocol for better prognostication of the disease. Moreover, VWI findings can guide antiviral treatment duration and helps to assess treatment response.
Abbreviations
- ACA:
-
Anterior cerebral artery
- BA:
-
Basilar artery
- CMV:
-
Cytomegalovirus
- COVID:
-
Corona virus disease
- FS:
-
Fat suppressed
- HBV:
-
Hepatitis B
- HCV:
-
Hepatitis C
- HSV:
-
Herpes simplex virus
- ICH:
-
Intracranial hemorrhage
- JE:
-
Japanese encephalitis
- MPRAGE:
-
Magnetization-prepared rapid gradient-echo
- PCA:
-
Posterior cerebral artery
- PCR:
-
Polymerase chain reaction
- RT:
-
Reverse transcriptase
- SARS-CoV2:
-
Severe acute respiratory syndrome coronavirus 2
- STA:
-
Superficial temporal artery
- VWI:
-
Vessel wall imaging
- VZV:
-
Varicella zoster virus
References
Nagel MA, Mahalingam R, Cohrs RJ, Gilden D (2010) Virus vasculopathy and stroke: an under-recognized cause and treatment target. Infect Disord Drug Targets 10(2):105–111. https://doi.org/10.2174/187152610790963537
South K, McCulloch L, McColl BW, Elkind MS, Allan SM, Smith CJ (2020) Preceding infection and risk of stroke: an old concept revived by the COVID-19 pandemic. Int J Stroke 15(7):722–732. https://doi.org/10.1177/1747493020943815
Wakamoto H, Ohta M, Nakano N, Kunisue K (2000) SPECT in focal enterovirus encephalitis: evidence for local cerebral vasculitis. Pediatr Neurol 23(5):429–431. https://doi.org/10.1016/S0887-8994(00)00206-X
Roden VJ, Cantor HE, O’Connor DM, Schmidt RR, Cherry JD (1975) Acute hemiplegia of childhood associated with Coxsackie A9 viral infection. J Pediatr 86(1):56–58. https://doi.org/10.1016/S0022-3476(75)80704-9
Castro Caldas A, Geraldes R, Neto L, Canhão P, Melo TP (2014) Central nervous system vasculitis associated with hepatitis C virus infection: a brain MRI-supported diagnosis. J Neurol Sci 336(1–2):152–154. https://doi.org/10.1016/j.jns.2013.10.028
Mandell DM, Mossa-Basha M, Qiao Y et al (2017) Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. Am J Neuroradiol 38(2):218–229. https://doi.org/10.3174/ajnr.A4893
Goeijenbier M, van Wissen M, van de Weg C et al (2012) Review: viral infections and mechanisms of thrombosis and bleeding. J Med Virol 84(10):1680–1696. https://doi.org/10.1002/jmv.23354
Hauer L, Pikija S, Schulte EC, Sztriha LK, Nardone R, Sellner J (2019) Cerebrovascular manifestations of herpes simplex virus infection of the central nervous system: a systematic review. J Neuroinflammation 16(1):19. https://doi.org/10.1186/s12974-019-1409-4
Fosse JH, Haraldsen G, Falk K, Edelmann R (2021) Endothelial cells in emerging viral infections. Front Cardiovasc Med 8:619690. https://doi.org/10.3389/fcvm.2021.619690
Choudhary N, Vyas S, Modi M et al (2021) MR vessel wall imaging in tubercular meningitis. Neuroradiology. https://doi.org/10.1007/s00234-021-02678-y
Cheng-Ching E, Jones S, Hui FK et al (2015) High-resolution MRI vessel wall imaging in varicella zoster virus vasculopathy. J Neurol Sci 351(1–2):168–173. https://doi.org/10.1016/j.jns.2015.02.017
Choudhary N, Vyas S, Ahuja CK et al (2021) MR vessel wall imaging in cerebral bacterial and fungal infections. Neuroradiology. https://doi.org/10.1007/s00234-021-02778-9
Uginet M, Breville G, Hofmeister J et al (2021) Cerebrovascular complications and vessel wall imaging in COVID-19 encephalopathy-a pilot study. Clin Neuroradiol. https://doi.org/10.1007/s00062-021-01008-2
Cheron J, Wyndham-Thomas C, Sadeghi N, Naeije G (2017) Response of human immunodeficiency virus-associated cerebral angiitis to the combined antiretroviral therapy. Front Neurol 8:95. https://doi.org/10.3389/fneur.2017.00095
Fan TH, Khoury J, Cho S-M, Bhimraj A, Shoskes A, Uchino K (2020) Cerebrovascular complications and vasculopathy in patients with herpes simplex virus central nervous system infection. J Neurol Sci 419:117200. https://doi.org/10.1016/j.jns.2020.117200
Askalan R, Laughlin S, Mayank S et al (2001) Chickenpox and stroke in childhood: a study of frequency and causation. Stroke 32(6):1257–1262. https://doi.org/10.1161/01.str.32.6.1257
Braun KPJ, Bulder MMM, Chabrier S et al (2009) The course and outcome of unilateral intracranial arteriopathy in 79 children with ischaemic stroke. Brain J Neurol 132(Pt 2):544–557. https://doi.org/10.1093/brain/awn313
Baskin HJ, Hedlund G (2007) Neuroimaging of herpesvirus infections in children. Pediatr Radiol 37(10):949–963. https://doi.org/10.1007/s00247-007-0506-1
Nagel MA, Cohrs RJ, Mahalingam R et al (2008) The varicella zoster virus vasculopathies: clinical, CSF, imaging, and virologic features. Neurology 70(11):853–860. https://doi.org/10.1212/01.wnl.0000304747.38502.e8
Tsivgoulis G, Lachanis S, Magoufis G, Safouris A, Kargiotis O, Stamboulis E (2016) High-resolution vessel wall magnetic resonance imaging in varicella-zoster virus vasculitis. J Stroke Cerebrovasc Dis 25(6):e74–e76. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.03.019
Wintermark M, Hills NK, DeVeber GA et al (2017) Clinical and imaging characteristics of arteriopathy subtypes in children with arterial ischemic stroke: results of the VIPS study. Am J Neuroradiol 38(11):2172–2179. https://doi.org/10.3174/ajnr.A5376
Nagel MA, Bubak AN (2018) Varicella zoster virus vasculopathy. J Infect Dis 218(suppl_2):S107–S112. https://doi.org/10.1093/infdis/jiy425
Connor MD, Lammie GA, Bell JE, Warlow CP, Simmonds P, Brettle RD (2000) Cerebral infarction in adult AIDS patients: observations from the Edinburgh HIV Autopsy Cohort. Stroke 31(9):2117–2126. https://doi.org/10.1161/01.str.31.9.2117
Alqaqa A (2017) HIV-Associated vasculopathy. In: Dumais N, ed. HIV/AIDS - Contemporary Challenges. InTech 43–54. https://doi.org/10.5772/66655
Mochan A, Modi M, Modi G (2003) Stroke in Black South African HIV-positive patients: a prospective analysis. Stroke 34(1):10–15. https://doi.org/10.1161/01.STR.0000043821.35051.FA
Arktout S (2020) Vessel wall MRI in HIV-associated cerebral angiitis. J Belg Soc Radiol 104(1):60. https://doi.org/10.5334/jbsr.2162
Mathew S, Pandian JD (2010) Stroke in patients with dengue. J Stroke Cerebrovasc Dis 19(3):253–256. https://doi.org/10.1016/j.jstrokecerebrovasdis.2009.05.003
Nanda SK, Jayalakshmi S, Mohandas S (2014) Pediatric ischemic stroke due to dengue vasculitis. Pediatr Neurol 51(4):570–572. https://doi.org/10.1016/j.pediatrneurol.2014.06.019
Kohlhaas K, Brechmann T, Vorgerd M (2007) Hepatitis-B-assoziierte Panarteriitis nodosa mit zerebraler Vaskulitis. DMW - Dtsch Med Wochenschr 132(34/35):1748–1752. https://doi.org/10.1055/s-2007-984960
Friede RL (1973) Cerebral infarcts complicating neonatal leptomeningitis: acute and residual lesions. Acta Neuropathol (Berl) 23(3):245–253. https://doi.org/10.1007/BF00687879
Chatterjee D, Radotra B, Vasishta R, Sharma K (2015) Vascular complications of tuberculous meningitis: an autopsy study. Neurol India 63(6):926. https://doi.org/10.4103/0028-3886.170086
Obusez EC, Hui F, Hajj-Ali RA et al (2014) High-resolution MRI vessel wall imaging: spatial and temporal patterns of reversible cerebral vasoconstriction syndrome and central nervous system vasculitis. AJNR Am J Neuroradiol 35(8):1527–1532. https://doi.org/10.3174/ajnr.A3909
Misra UK, Kalita J, Nair PP (2010) Role of aspirin in tuberculous meningitis: a randomized open label placebo controlled trial. J Neurol Sci 293(1–2):12–7. https://doi.org/10.1016/j.jns.2010.03.025
Author information
Authors and Affiliations
Contributions
Sameer Vyas: Project development, Concept Designing, Data Collection, Manuscript writing
Neha Choudhary: Project development, Concept Designing, Data Collection, Manuscript writing
Manish Modi: Data Collection, Manuscript writing and patients care
Naveen Sankhyan: Data Collection, Manuscript writing and patients care
Renu Suthar: Data Collection, Manuscript writing and patients care
Arushi Gahlot Saini: Data Collection, Manuscript writing and patients care
Arun Bansal: Data Collection, Manuscript writing and patients care
Navneet Sharma: Data Collection, Manuscript writing and patients care
Paramjeet Singh: Data Collection, Manuscript writing
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vyas, S., Choudhary, N., Modi, M. et al. High-resolution intracranial vessel wall imaging in cerebral viral infections evaluations. Neuroradiology 64, 915–924 (2022). https://doi.org/10.1007/s00234-021-02831-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02831-7