Abstract
Incidental basal ganglia calcifications are a common finding on computed tomography (CT). We investigated the histological characteristics of these calcifications and their association with CT findings, using post-mortem basal ganglia tissue from 22 patients. Eight patients had basal ganglia calcifications on histology, and six patients had calcifications on CT, varying from mild to severe. Four patients had calcifications identified by both histology and CT, and two patients had calcifications detected by CT but not by histology, possibly because of insufficient tissue available. Calcifications were found mainly in the tunica media of arterioles located in the globus pallidus, which suggests that incidental CT calcifications are vascular in nature. However, tunica media calcifications, and thereby incidental basal ganglia calcifications, are probably not related to atherosclerosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first radiological description of basal ganglia calcifications dates from 1924 [1]. Nowadays, calcifications in the basal ganglia are most often detected incidentally during computed tomography (CT) scanning of the brain and have a prevalence of 0.32–38% [2,3,4]. These calcifications are usually considered innocent, although they may be associated with diabetes and psychotic symptoms [4, 5]. Patients with Fahr disease, who have severe basal ganglia calcification, suffer from movement disorders, cognitive disorders, and psychiatric symptoms [6]. This suggests that basal ganglia calcifications may not be so harmless. In case reports of patients with Fahr disease, the calcifications occurred in capillaries and the tunica media of arterioles and small- and medium-caliber arteries, with large arteries and veins sometimes showing complete calcification of the vessel wall [7, 8]. To our knowledge, there is one neuropathological study describing the histological nature of incidental basal ganglia calcifications in patients who did not have Fahr disease but who had a neurodegenerative disease, such as Alzheimer’s disease, frontotemporal dementia, progressive supranuclear palsy, or Parkinson’s disease [9]. The study described three patterns of calcification: deposits within the tunica media, deposits in the parenchyma, and deposits along capillaries. Calcifications in the internal elastic lamina and tunica media are usually non-atherosclerotic in origin, in contrast to calcifications of the tunica intima, and are associated with diabetes mellitus and chronic kidney disease [10].
We investigated the histological nature of incidental basal ganglia calcifications and whether histological findings are associated with CT findings.
Methods
Local ethical committee approval was obtained for research on retained tissues after written informed consent was given by the patients during life or their next of kin after death (Medical Ethics Committee of the **** 11-531/C). Between 1-1-2013 and 31-12-2018, we identified 22 adult patients for whom there were unenhanced CT scans of the brain (at maximum 1 year before autopsy) and brain autopsy findings available. Histological findings were compared to a consensus CT calcification score.
CT scans
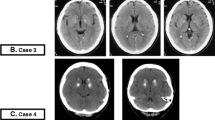
The 22 unenhanced CT scans of the brain were anonymized. They were acquired on Philips Brilliance 64-slice to 256-slice CT scanners (Philips Healthcare, Best, The Netherlands) and reconstructed in thin slices (max 1 millimeter). Basal ganglia calcifications were scored as absent, mild (one dot), moderate (multiple dots or a single artery), or severe (confluent) (Fig. 1) [4]. Three experienced radiologists (***, ***, ***) blinded to the histological report scored all the scans together in a consensus meeting, using the Philips IntelliSpace Portal 7.0 (Philips Healthcare, Best, The Netherlands) in the brain window setting (center 40 Hounsfield units, width 80 Hounsfield units) and axial, coronal, and sagittal views.
Histology
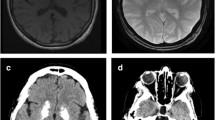
The basal ganglia were sampled routinely and usually divided into two formalin-fixed, paraffin-embedded blocks covering the entire central grey matter up to the insular cortex. Sections were stained with hematoxylin and eosin (Fig. 2a). An experienced neuropathologist (***), who did not know the CT findings, scored the calcifications with a newly developed method as follows: mild calcification was defined as granular to fine lamellar, more often diffuse and fuchsia to purple staining; moderate calcification was defined as linear, incomplete (semi/noncircular), and deep purple staining; and severe calcification was defined as linear, circular, and deep purple to dark blue staining (Fig. 2b–d). Besides severity, the extent of calcification was assessed as being discrete/limited (sporadic), moderate (the minority), and extensive (the majority). The anatomical distribution of calcifications, i.e., globus pallidus interna, globus pallidus externa, and putamen, was assessed.
Results
The 22 subjects (11 males) were 22–92 years old (median 69 years). On CT, six patients had calcifications, with calcification being severe in one patient, moderate in one patient, and mild in four patients (Table 1). On histology, eight patients had basal ganglia calcifications located in the tunica media of arterioles (Table 1). The severity of the calcifications was mild in three patients, moderate in three patients, and severe in two patients. The extent of the calcifications was assessed as discrete/limited in two patients, moderate in three patients, and extensive in three patients. The calcifications were localized in the internal globus pallidus in all patients and additionally in the external globus pallidus in six patients. Calcification of the putamen was not seen.
Comparison of the histological and CT findings showed that histologically proven calcifications were also seen on CT in four patients, whereas mild calcifications detected on CT were not detected histologically in two patients.
On closer inspection, the deposits in the vessel wall seemed to arise along the internal elastic lamina as granular to linear deposits in an early stage, merging with more peripheral calcifications in or along the media in a later stage, ultimately forming a single (semi-) circular deposit (Fig. 2d). This distribution pattern started in the ventral striatopallidum and fanned out posterolaterally into the external half of the globus pallidus, seemingly following the vascular tree downstream (Fig. 2a).
Discussion
We investigated the histological nature of incidental basal ganglia calcifications and their association with CT findings. On histology, in an early stage, calcification was detected as granular to linear deposits in the internal elastic lamina, merging with more peripheral calcifications in or along the media in a later stage, ultimately forming a single (semi-) circular deposit. This is consistent with the type 1 calcifications described by Fujita [9] in patients with neurodegenerative disease and also consistent with findings in patients with Fahr disease [7, 8].
This study adds new information about calcifications in patients without Fahr disease, in group patients who underwent brain autopsy after their death.
CT scanning is the most common method to detect calcifications in the basal ganglia. However, comparison of CT and histological findings is problematic, because CT may not be sensitive enough to detect small calcifications. This is probably why CT scanning did not detect histologically proven calcifications in four patients. A limitation of histology is that sampling may miss areas with calcifications that are seen on CT, which may explain why two patients had calcifications seen on CT but not confirmed by histology. Nevertheless, in four patients, the basal ganglia calcifications seen on CT correlated with histological findings, namely, calcifications in small- and medium-sized vessels, probably following the vascular tree downstream. As the calcifications were located in the internal elastic lamina and the tunica media, we conclude that incidental CT calcifications are vascular in nature, but probably not atherosclerotic. The relevance of incidental basal ganglia calcifications needs to be investigated and correlated with motor, cognitive, and psychiatric symptoms, such as those seen in Fahr disease.
References
Fritzsche R (1924) Eine familiar auftrentende From vonolidophrenie mit vontgenologish nachveisbaren symmetrischein Kalkoablagerungenim Geheirn besounders in den Stammganglien Schweiz. Arch Neurol Neurochir Psychiatr 1:29–33
Simoni M, Pantoni L, Pracucci G, Palmertz B, Guo X, Gustafson D, Skoog I (2008) Prevalence of CT-detected cerebral abnormalities in an elderly Swedish population sample. Acta Neurol Scand 118:260–267
Koller WC, Cochran JW, Klawans HL (1979) Calcification of the basal ganglia: computerized tomography and clinical correlation. Neurology 29:328–333
De Brouwer EJM, Kockelkoren R, De Vis JB, Dankbaar JW, Velthuis BK, Takx RAP, De Jonghe A, Emmelot-Vonk MH, Koek HL, De Jong PA (2020) Prevalence and vascular risk factors of basal ganglia calcifications in patients at risk for cerebrovascular disease. JNeuroradiol 47:339–344. https://doi.org/10.1016/j.neurad.2019.04.002
Ostling S, Andreasson LA, Skoog I (2003) Basal ganglia calcification and psychotic symptoms in the very old. Int J Geriatr Psychiatry 18:983–987
Manyam BV (2005) What is and what is not ‘Fahr’s disease. Parkinsonism Relat Disord 11:73–80
Miklossy J, Mackenzie IR, Dorovini-Zis K, Calne DB, Wszolek ZK, Klegeris A, McGeer PL (2005) Severe vascular disturbance in a case of familial brain calcinosis. Acta Neuropathol 109:643–653
Kimura T, Miura T, Aoki K, Saito S, Hondo H, Konno T, Uchiyama A, Ikeuchi T, Takahashi H, Kakita A (2016) Familial idiopathic basal ganglia calcification: histopathologic features of an autopsied patient with an SLC20A2 mutation. Neuropathology 36:365–371
Fujita D, Terada S, Ishizu H, Yokota O, Nakashima H, Ishihara T, Kuorda S (2003) Immunohistochemical examination on intracranial calcification in neurodegenerative diseases. Acta Neuropathol 105:259–264. https://doi.org/10.1007/s00401-002-0640-7
Bartstra JW, van den Beukel TC, Van Hecke W, Mali WPTM, Spiering W, Koek HL, Hendrikse J, de Jong PA, den Harder AM (2020) Intracranial arterial calcification: prevalence, risk factors, and consequences. J Am Coll Cardiol 76(13):1595–1604. https://doi.org/10.1016/j.jacc.2020.07.056
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was given by the patients during life or their next of kin after death.
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Brouwer, E.J.M., de Jong, P.A., De Jonghe, A. et al. Histology and computed tomography of incidental calcifications in the human basal ganglia. Neuroradiology 63, 1145–1148 (2021). https://doi.org/10.1007/s00234-021-02680-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02680-4