Abstract
Purpose
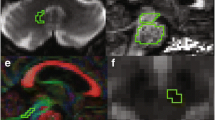
The word “fixel” refers to the specific fiber population within each voxel, and fixel-based analysis (FBA) is a recently developed technique that facilitates fiber tract-specific statistical analysis. The aim of the paper is to apply FBA to detect impaired fibers for corticobasal syndrome (CBS) especially in regions that contain multiple crossed fibers.
Methods
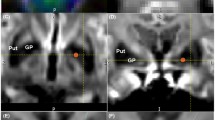
FBA was performed in cohorts of participants clinically diagnosed with CBS (n = 10) and Parkinson’s disease (n = 15) or in healthy controls (n = 9). The parameters of the diffusion weighted image were echo time, 83 ms; time, 8123.6 ms; flip angle, 90°; section thickness, 2 mm; b = 1000 s/mm2; and 32 axes. Diffusion tensor analysis was conducted using tract-based spatial statistics (TBSS), and white matter volume was estimated via voxel-based morphometry.
Results
A comparison of PD or HC to CBS revealed a significant difference in the dentatorubrothalamic tract of the brainstem in FBA in addition to the affected regions in voxel-based morphometry and TBSS (family-wise error-corrected p < 0.05). Reduction of the white matter fibers crossing the brainstem could not be detected via microstructural changes identified using TBSS, but it was detected using FBA.
Conclusion
FBA has some advantages in determining the distribution of corticobasal syndrome lesions.



Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Boelmans K, Bodammer NC, Suchorska B, Kaufmann J, Ebersbach G, Heinze HJ, Niehaus L (2010) Diffusion tensor imaging of the corpus callosum differentiates corticobasal syndrome from Parkinson's disease. Parkinsonism Relat Disord 16:498–502
Quattrone A, Caligiuri ME, Morelli M, Nigro S, Vescio B, Arabia G, Nicoletti G, Nisticò R, Salsone M, Novellino F, Barbagallo G, Vaccaro MG, Sabatini U, Vescio V, Stanà C, Rocca F, Caracciolo M (2019) Imaging counterpart of postural instability and vertical ocular dysfunction in patients with PSP: A multimodal MRI study. Parkinsonism Relat Disord 63:124–130
Schwarz CG, Reid RI, Gunter JL, Senjem ML, Przybelski SA, Zuk SM, Whitwell JL, Vemuri P, Josephs KA, Kantarci K, Thompson PM, Petersen RC, Jack CR, Alzheimer’s Disease Neuroimaging Initiative (2014) Improved DTI registration allows voxel-based analysis that outperforms tract-based spatial statistics. Neuroimage 94:65–78
Surova Y, Nilsson M, Lätt J, Lampinen B, Lindberg O, Hall S, Widner H, Nilsson C, van Westen D, Hansson O (2015) Disease-specific structural changes in thalamus and dentatorubrothalamic tract in progressive supranuclear palsy. Neuroradiology 57:1079–1091
Worker A, Blain C, Jarosz J, Chaudhuri KR, Barker GJ, Williams SC, Brown RG, Leigh PN, Dell’Acqua F, Simmons A (2014) Diffusion tensor imaging of Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy: a tract-based spatial statistics study. PLoS One 9:e112638
Zanigni S, Evangelisti S, Testa C, Manners DN, Calandra-Buonaura G, Guarino M, Gabellini A, Gramegna LL, Giannini G, Sambati L, Cortelli P, Lodi R, Tonon C (2017) White matter and cortical changes in atypical parkinsonisms: a multimodal quantitative MR study. Parkinsonism Relat Disord 39:44–51
Jeurissen B, Leemans A, Tournier JD, Jones DK, Sijbers J (2013) Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp 34:2747–2766
Raffelt DA, Smith RE, Ridgway GR, Tournier JD, Vaughan DN, Rose S, Henderson R, Connelly A (2015) Connectivity-based fixel enhancement: Whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. Neuroimage 117:40–55
Vaughan DN, Raffelt D, Curwood E, Tsai MH, Tournier JD, Connelly A, Jackson GD (2017) Tract-specific atrophy in focal epilepsy: disease, genetics, or seizures? Ann Neurol 81:240–250
Mito R, Raffelt D, Dhollander T, Vaughan DN, Tournier JD, Salvado O, Brodtmann A, Rowe CC, Villemagne VL, Connelly A (2018) Fibre-specific white matter reductions in Alzheimer’s disease and mild cognitive impairment. Brain 141:888–902
Gajamange S, Raffelt D, Dhollander T, Lui E, van der Walt A, Kilpatrick T, Fielding J, Connelly A, Kolbe S (2018) Fibre-specific white matter changes in multiple sclerosis patients with optic neuritis. Neuroimage Clin 17:60–68
Grazioplene RG, Bearden CE, Subotnik KL, Ventura J, Haut K, Nuechterlein KH, Cannon TD (2018) Connectivity-enhanced diffusion analysis reveals white matter density disruptions in first episode and chronic schizophrenia. Neuroimage Clin 18:608–616
Mu J, Chen T, Li P, Ding D, Ma X, Zhang M, Liu J (2018) Altered white matter microstructure mediates the relationship between hemoglobin levels and cognitive control deficits in end-stage renal disease patients. Hum Brain Mapp 39:4766–4775
Adanyeguh IM, Perlbarg V, Henry PG, Rinaldi D, Petit E, Valabregue R, Brice A, Durr A, Mochel F (2018) Autosomal dominant cerebellar ataxias: imaging biomarkers with high effect sizes. Neuroimage Clin 19:858–867
Lyon M, Welton T, Varda A, Maller JJ, Broadhouse K, Korgaonkar MS, Koslow SH, Williams LM, Gordon E, Rush AJ, Grieve SM (2019) Gender-specific structural abnormalities in major depressive disorder revealed by fixel-based analysis. Neuroimage Clin 21:101668
Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Tröster AI, Vidailhet M, Weiner WJ (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80:496–503
Ishizawa K, Dickson DW (2001) Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. J Neuropathol Exp Neurol 60:647–657
Padovani A, Borroni B, Brambati SM, Agosti C, Broli M, Alonso R, Scifo P, Bellelli G, Alberici A, Gasparotti R, Perani D (2006) Diffusion tensor imaging and voxel based morphometry study in early progressive supranuclear palsy. J Neurol Neurosurg Psychiatry 77:457–463
Tsuboi Y, Slowinski J, Josephs KA, Honer WG, Wszolek ZK, Dickson DW (2003) Atrophy of superior cerebellar peduncle in progressive supranuclear palsy. Neurology 60:1766–1769
Whitwell JL, Master AV, Avula R, Kantarci K, Eggers SD, Edmonson HA, Jack CR Jr, Josephs KA (2011) Clinical correlates of white matter tract degeneration in progressive supranuclear palsy. Arch Neurol 68:753–760
Daniel SE, Lees AJ (1993) Parkinson’s Disease Society Brain Bank, London: overview and research. J Neural Transm Suppl 39:165–172
Helenius J, Goddeau RP Jr, Moonis M, Henninger N (2016) Impact of leukoaraiosis burden on hemispheric lateralization of the National Institutes of Health Stroke Scale deficit in acute ischemic stroke. Stroke 47:24–30
Raffelt DA, Tournier JD, Smith RE, Vaughan DN, Jackson G, Ridgway GR, Connelly A (2017) Investigating white matter fibre density and morphology using fixel-based analysis. Neuroimage 144:58–73
Piattella MC, Upadhyay N, Bologna M, Sbardella E, Tona F, Formica A, Petsas N, Berardelli A, Pantano P (2015) Neuroimaging evidence of gray and white matter damage and clinical correlates in progressive supranuclear palsy. J Neurol 262:1850–1858
Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Jack CR Jr (2008) Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging 29:280–289
Sakurai K, Imabayashi E, Tokumaru AM, Hasebe S, Murayama S, Morimoto S, Kanemaru K, Takao M, Shibamoto Y, Matsukawa N (2015) The feasibility of white matter volume reduction analysis using SPM8 plus DARTEL for the diagnosis of patients with clinically diagnosed corticobasal syndrome and Richardson's syndrome. Neuroimage Clin 7:605–610
Wolpe N, Moore JW, Rae CL, Rittman T, Altena E, Haggard P, Rowe JB (2014) The medial frontal-prefrontal network for altered awareness and control of action in corticobasal syndrome. Brain 137:208–220
Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, Weiner MW, Rosen HJ (2006) Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol 63:81–86
Burrell JR, Hornberger M, Vucic S, Kiernan MC, Hodges JR (2014) Apraxia and motor dysfunction in corticobasal syndrome. PLoS One 9:e92944
Jütten K, Pieperhoff P, Südmeyer M, Schleicher A, Ferrea S, Caspers S, Zilles K, Schnitzler A, Amunts K, Lux S (2014) Neuropsychological and brain volume differences in patients with left- and right-beginning corticobasal syndrome. PLoS One 9:e110326
Whitwell JL, Jack CR Jr, Boeve BF, Parisi JE, Ahlskog JE, Drubach DA, Senjem ML, Knopman DS, Petersen RC, Dickson DW, Josephs KA (2010) Imaging correlates of pathology in corticobasal syndrome. Neurology 75:1879–1887
Yu F, Barron DS, Tantiwongkosi B, Fox P (2015) Patterns of gray matter atrophy in atypical parkinsonism syndromes: a VBM meta-analysis. Brain Behav 5:e00329
Di Stasio F, Suppa A, Marsili L, Upadhyay N, Asci F, Bologna M, Colosimo C, Fabbrini G, Pantano P, Berardelli A (2019) Corticobasal syndrome: neuroimaging and neurophysiological advances. Eur J Neurol 26:701–e52
Erbetta A, Mandelli ML, Savoiardo M, Grisoli M, Bizzi A, Soliveri P, Chiapparini L, Prioni S, Bruzzone MG, Girotti F (2009) Diffusion tensor imaging shows different topographic involvement of the thalamus in progressive supranuclear palsy and corticobasal degeneration. AJNR Am J Neuroradiol 30:1482–1487
Upadhyay N, Suppa A, Piattella MC, Bologna M, Di Stasio F, Formica A, Tona F, Colosimo C, Berardelli A, Pantano P (2016) MRI gray and white matter measures in progressive supranuclear palsy and corticobasal syndrome. J Neurol 263:2022–2031
Zhang Y, Walter R, Ng P, Luong PN, Dutt S, Heuer H, Rojas-Rodriguez JC, Tsai R, Litvan I, Dickerson BC, Tartaglia MC, Rabinovici G, Miller BL, Rosen HJ, Schuff N, Boxer AL (2016) Progression of microstructural degeneration in progressive supranuclear palsy and corticobasal syndrome: a longitudinal diffusion tensor imaging study. PLoS One 11:e0157218
Whitwell JL, Schwarz CG, Reid RI, Kantarci K, Jack CR Jr, Josephs KA (2014) Diffusion tensor imaging comparison of progressive supranuclear palsy and corticobasal syndromes. Parkinsonism Relat Disord 20:493–498
Kwon HG, Hong JH, Hong CP, Lee DH, Ahn SH, Jang SH (2011) Dentatorubrothalamic tract in human brain: diffusion tensor tractography study. Neuroradiology 53:787–791
Alexander SK, Rittman T, Xuereb JH, Bak TH, Hodges JR, Rowe JB (2014) Validation of the new consensus criteria for the diagnosis of corticobasal degeneration. J Neurol Neurosurg Psychiatry 85:925–929
Ali F, Josephs KA (2018) Corticobasal degeneration: key emerging issues. J Neurol 265:439–445
Upadhyay N, Suppa A, Piattella MC, Di Stasio F, Petsas N, Colonnese C, Colosimo C, Berardelli A, Pantano P (2016) Gray and white matter structural changes in corticobasal syndrome. Neurobiol Aging 37:82–90
Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS, Tabaton M, Vonsattel JP, Wakabayashi K, Litvan I, Office of Rare Diseases of the National Institutes of Health (2002) Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–946
Wilkins B, Lee N, Gajawelli N, Law M, Leporé N (2015) Fiber estimation and tractography in diffusion MRI: development of simulated brain images and comparison of multi-fiber analysis methods at clinical b-values. Neuroimage 109:341–356
Tournier JD, Calamante F, Connelly A (2007) Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 35:1459–1472
Andersson JL, Skare S, Ashburner J (2003) How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20:870–888
Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J (2014) Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 103:411–426
Caligiuri ME, Perrotta P, Augimeri A, Rocca F, Quattrone A, Cherubini A (2015) Automatic detection of white matter hyperintensities in healthy aging and pathology using magnetic resonance imaging: a review. Neuroinformatics 13:261–276
Acknowledgments
We thank the patients, researchers, and clinicians involved in the Hyogo College of Medicine and Dr. Igeta for advice on statistical data.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 706 kb)
Rights and permissions
About this article
Cite this article
Sakamoto, S., Kimura, T., Kajiyama, K. et al. Dentatorubrothalamic tract reduction using fixel-based analysis in corticobasal syndrome. Neuroradiology 63, 529–538 (2021). https://doi.org/10.1007/s00234-020-02559-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02559-w