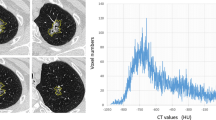
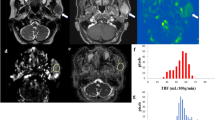
Abstract
Purpose
We compared the diagnostic performance of CT texture analysis in single-phase CT scan with that of conventional enhancement pattern analysis in a two-phase CT scan for discrimination of salivary gland tumors, Warthin tumor (WT) from pleomorphic adenoma (PA).
Methods
One hundred seventy-eight patients with PA and 84 patients with WT were selected and CT texture analysis was separately performed on early (40s) and delayed (180s) phases, after injection of the contrast agent, using commercially available software. The attenuation changes and enhancement patterns were visually and quantitatively assessed with Hounsfield units (HU). Differences between PAs and WTs were analyzed using χ2 test and independent t test. Diagnostic performance of texture parameters in single-phase CT was compared with that of dynamic enhancement pattern in two-phase CT using the McNemar test.
Results
Ratio of tumoral HU (delayed phase/early phase) was significantly higher in PAs compared with WTs (p < 0.001). Tumor heterogeneity parameters, standard deviation (SD) and entropy, were significantly lower in WTs regardless of the type of filter used (p ≤ 0.001). Mean with coarse filter (AUC = 0.944) on early phase scan and entropy with medium filter (AUC = 0.901) on delayed scan were best discriminators between PAs and WTs. Diagnostic accuracy of mean (90.5%) on early scan and entropy (84.7%) on delayed scan was not significantly different from the accuracy (89.3%) of conventional wash-out pattern for distinguishing WTs from PAs (p = 0.742, p = 0.088, respectively).
Conclusion
Diagnostic performance of texture parameters was similar to that of quantitative enhancement pattern for differentiating WTs from PAs, with the advantage in lower radiation exposure.

Similar content being viewed by others
References
Pinkston JA, Cole P (1999) Incidence rates of salivary gland tumors: results from a population-based study. Otolaryngol Head Neck Surg 120:834–840
Araya J, Martinez R, Niklander S, Marshall M, Esguep A (2015) Incidence and prevalence of salivary gland tumours in Valparaiso, Chile. Med Oral Patol Oral Cir Bucal 20:e532–e539
Koch M, Zenk J, Iro H (2010) Long-term results of morbidity after parotid gland surgery in benign disease. Laryngoscope 120:724–730
Wong WK, Shetty S (2018) The extent of surgery for benign parotid pathology and its influence on complications: a prospective cohort analysis. Am J Otolaryngol 39:162–166
Thoeny HC (2007) Imaging of salivary gland tumours. Cancer Imaging 7:52–62
Kwon KH, Suh JH, Hur MH, Chung WY, Kang HY, Park CS (2001) Enucleation for the management of the parotid Warthin's tumor. J Korean Surg Soc 61:474–478
Heller KS, Attie JN (1988) Treatment of Warthin's tumor by enucleation. Am J Surg 156:294–296
Joo YH, Kim JP, Park JJ, Woo SH (2014) Two-phase helical computed tomography study of salivary gland Warthin tumors: a radiologic findings and surgical applications. Clin Exp Otorhinolaryngol 7:216–221
Schindler S, Nayar R, Dutra J, Bedrossian CW (2001) Diagnostic challenges in aspiration cytology of the salivary glands. Semin Diagn Pathol 18:124–146
Schmidt RL, Hall BJ, Wilson AR, Layfield LJ (2011) A systematic review and meta-analysis of the diagnostic accuracy of fine-needle aspiration cytology for parotid gland lesions. Am J Clin Pathol 136:45–59
Seethala RR, LiVolsi VA, Baloch ZW (2005) Relative accuracy of fine-needle aspiration and frozen section in the diagnosis of lesions of the parotid gland. Head Neck 27:217–223
Lee YY, Wong KT, King AD, Ahuja AT (2008) Imaging of salivary gland tumours. Eur J Radiol 66:419–436
Miao LY, Xue H, Ge HY, Wang JR, Jia JW, Cui LG (2015) Differentiation of pleomorphic adenoma and Warthin’s tumour of the salivary gland: is long-to-short diameter ratio a useful parameter? Clin Radiol 70:1212–1219
Liu Y, Li J, Tan YR, Xiong P, Zhong LP (2015) Accuracy of diagnosis of salivary gland tumors with the use of ultrasonography, computed tomography, and magnetic resonance imaging: a meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol 119(238–245):e232
Aghaghazvini L, Salahshour F, Yazdani N, Sharifian H, Kooraki S, Pakravan M, Shakiba M (2015) Dynamic contrast-enhanced MRI for differentiation of major salivary glands neoplasms, a 3-T MRI study. Dentomaxillofac Radiol 44:20140166
Habermann CR, Arndt C, Graessner J, Diestel L, Petersen KU, Reitmeier F, Ussmueller JO, Adam G, Jaehne M (2009) Diffusion-weighted echo-planar MR imaging of primary parotid gland tumors: is a prediction of different histologic subtypes possible? AJNR Am J Neuroradiol 30:591–596
Matsusue E, Fujihara Y, Matsuda E, Tokuyasu Y, Nakamoto S, Nakamura K, Ogawa T (2017) Differentiating parotid tumors by quantitative signal intensity evaluation on MR imaging. Clin Imaging 46:37–43
Yabuuchi H, Fukuya T, Tajima T, Hachitanda Y, Tomita K, Koga M (2003) Salivary gland tumors: diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology 226:345–354
Choi DS, Na DG, Byun HS, Ko YH, Kim CK, Cho JM, Lee HK (2000) Salivary gland tumors: evaluation with two-phase helical CT. Radiology 214:231–236
Yerli H, Aydin E, Coskun M, Geyik E, Ozluoglu LN, Haberal N, Kaskati T (2007) Dynamic multislice computed tomography findings for parotid gland tumors. J Comput Assist Tomogr 31:309–316
Woo SH, Choi DS, Kim JP, Park JJ, Joo YH, Chung PS, Kim BY, Ko YH, Jeong HS, Kim HJ (2013) Two-phase computed tomography study of Warthin tumor of parotid gland: differentiation from other parotid gland tumors and its pathologic explanation. J Comput Assist Tomogr 37:518–524
Howlett DC, Kesse KW, Hughes DV, Sallomi DF (2002) The role of imaging in the evaluation of parotid disease. Clin Radiol 57:692–701
Lubner MG, Smith AD, Sandrasegaran K, Sahani DV, Pickhardt PJ (2017) CT texture analysis: definitions, applications, biologic correlates, and challenges. Radiographics 37:1483–1503
Tsai A, Buch K, Fujita A, Qureshi MM, Kuno H, Chapman MN, Li B, Oda M, Truong MT, Sakai O (2018) Using CT texture analysis to differentiate between nasopharyngeal carcinoma and age-matched adenoid controls. Eur J Radiol 108:208–214
Buch K, Fujita A, Li B, Kawashima Y, Qureshi MM, Sakai O (2015) Using texture analysis to determine human papillomavirus status of oropharyngeal squamous cell carcinomas on CT. AJNR Am J Neuroradiol 36:1343–1348
Kuno H, Qureshi MM, Chapman MN, Li B, Andreu-Arasa VC, Onoue K, Truong MT, Sakai O (2017) CT texture analysis potentially predicts local failure in head and neck squamous cell carcinoma treated with chemoradiotherapy. AJNR Am J Neuroradiol 38:2334–2340
Lee JY, Han M, Kim KS, Shin SJ, Choi JW, Ha EJ (2019) Discrimination of HPV status using CT texture analysis: tumour heterogeneity in oropharyngeal squamous cell carcinomas. Neuroradiology 61:1415–1424
Seifert G, Miehlke A, Haubrich J, Chilla R (1986) Diseases of the salivary glands. Thieme, Stuttgart
Gnepp DR, El-Mofty SK (1996) Salivary gland. In: Damjanov I, Linder J (eds) Anderson's pathology, 10th edn. Mosby, St Louis
Miles KA, Ganeshan B, Hayball MP (2013) CT texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging 13:400–406
Ganeshan B, Miles KA (2013) Quantifying tumour heterogeneity with CT. Cancer Imaging 13:140–149
Sakamoto M, Sasano T, Higano S, Takahashi S, Iikubo M, Kakehata S (2003) Usefulness of heavily T2 weighted magnetic resonance images for the differential diagnosis of parotid tumours. Dentomaxillofac Radiol 32:295–299
Ryoo I, Suh S, Lee YH, Seo HS, Seol HY, Woo JS, Kim SC (2018) Vascular pattern analysis on microvascular Sonography for differentiation of pleomorphic adenomas and Warthin tumors of salivary glands. J Ultrasound Med 37:613–620
Kim TY, Lee Y (2019) Contrast-enhanced multi-detector CT examination of parotid gland tumors: determination of the Most helpful scanning delay for predicting histologic subtypes. J Belg Soc Radiol 103:2
Hisatomi M, Asaumi J, Yanagi Y, Konouchi H, Matsuzaki H, Honda Y, Kishi K (2003) Assessment of pleomorphic adenomas using MRI and dynamic contrast enhanced MRI. Oral Oncol 39:574–579
Ikeda M, Motoori K, Hanazawa T, Nagai Y, Yamamoto S, Ueda T, Funatsu H, Ito H (2004) Warthin tumor of the parotid gland: diagnostic value of MR imaging with histopathologic correlation. AJNR Am J Neuroradiol 25:1256–1262
Larue RT, Defraene G, De Ruysscher D, Lambin P, van Elmpt W (2017) Quantitative radiomics studies for tissue characterization: a review of technology and methodological procedures. Br J Radiol 90:20160665
Traverso A, Wee L, Dekker A, Gillies R (2018) Repeatability and reproducibility of radiomic features: a systematic review. Int J Radiat Oncol Biol Phys 102:1143–1158
Al Ajmi E, Forghani B, Reinhold C, Bayat M, Forghani R (2018) Spectral multi-energy CT texture analysis with machine learning for tissue classification: an investigation using classification of benign parotid tumours as a testing paradigm. Eur Radiol 28:2604–2611
Ng F, Kozarski R, Ganeshan B, Goh V (2013) Assessment of tumor heterogeneity by CT texture analysis: can the largest cross-sectional area be used as an alternative to whole tumor analysis? Eur J Radiol 82:342–348
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this retrospective study, formal consent is not required.
Informed consent
Written informed consent was waived by the Institutional Review Board. Institutional Review Board approval was obtained (AJIRB-MED-MDB-19-137).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 23 kb).
Rights and permissions
About this article
Cite this article
Jung, Y.J., Han, M., Ha, E.J. et al. Differentiation of salivary gland tumors through tumor heterogeneity: a comparison between pleomorphic adenoma and Warthin tumor using CT texture analysis. Neuroradiology 62, 1451–1458 (2020). https://doi.org/10.1007/s00234-020-02485-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02485-x