Abstract
Purpose
A new form of autosomal dominant hereditary spinocerebellar ataxia (SCA) has been recently described (SCA48), and here we investigate its conventional MRI findings to identify the presence of a possible imaging feature of this condition.
Methods
In this retrospective observational study, we evaluated conventional MRI scans from 10 SCA48 patients (M/F = 5/5; 44.7 ± 7.8 years). For all subjects, atrophy of both supratentorial and infratentorial compartments were recorded, as well as the presence of possible T2-weighted imaging (T2WI) signal alterations.
Results
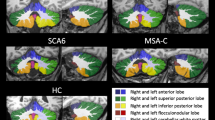
In SCA48 patients, no meaningful supratentorial changes were found, both in terms of volume loss or MRI signal changes. Atrophy of the cerebellum was present in all cases, involving both the vermis and the hemispheres, but particularly affecting the postero-lateral portions of the cerebellar hemispheres. In all patients, with the exception of only one subject (90.0% of the cases), a T2WI hyperintensity of both dentate nuclei was found. The association of such signal alteration with the pattern of cerebellar atrophy resembled the appearance of a crab (“crab sign”).
Conclusion
Our findings suggest that SCA48 patients are characterized by cerebellar atrophy, mainly involving the postero-lateral hemisphere areas, along with a T2WI hyperintensity of dentate nuclei. We propose that the association of such signal change, along with the atrophy of the lateral portion of the cerebellar hemispheres, resembled the appearance of a crab, and therefore, we propose the “crab sign” as a neuroradiological sign present in SCA48 patients.


Similar content being viewed by others
References
Sullivan R, Yau WY, O'Connor E, Houlden H (2019) Spinocerebellar ataxia: an update. J Neurol 266(2):533–544. https://doi.org/10.1007/s00415-018-9076-4
Durr A (2010) Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol 9(9):885–894. https://doi.org/10.1016/S1474-4422(10)70183-6
Ruano L, Melo C, Silva MC, Coutinho P (2014) The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology 42(3):174–183. https://doi.org/10.1159/000358801
De Michele G, Lieto M, Galatolo D, Salvatore E, Cocozza S, Barghigiani M, Tessa A, Baldacci J, Pappata S, Filla A, Santorelli FM (2019) Spinocerebellar ataxia 48 presenting with ataxia associated with cognitive, psychiatric, and extrapyramidal features: a report of two Italian families. Parkinsonism Relat Disord 65:91–96. https://doi.org/10.1016/j.parkreldis.2019.05.001
Genis D, Ortega-Cubero S, San Nicolas H, Corral J, Gardenyes J, de Jorge L, Lopez E, Campos B, Lorenzo E, Tonda R, Beltran S, Negre M, Obon M, Beltran B, Fabregas L, Alemany B, Marquez F, Ramio-Torrenta L, Gich J, Volpini V, Pastor P (2018) Heterozygous STUB1 mutation causes familial ataxia with cognitive affective syndrome (SCA48). Neurology 91(21):e1988–e1998. https://doi.org/10.1212/WNL.0000000000006550
Lieto M, Riso V, Galatolo D, De Michele G, Rossi S, Barghigiani M, Cocozza S, Pontillo G, Trovato R, Sacca F, Salvatore E, Tessa A, Filla A, Santorelli FM, Silvestri G (2019) The complex phenotype of spinocerebellar ataxia type 48 in eight unrelated Italian families. Eur J Neurol. https://doi.org/10.1111/ene.14094
Palvadeau R, Kaya-Gulec ZE, Simsir G, Vural A, Oztop-Cakmak O, Genc G, Aygun MS, Falay O, Basak AN, Ertan S (2019) Cerebellar cognitive-affective syndrome preceding ataxia associated with complex extrapyramidal features in a Turkish SCA48 family. Neurogenetics. 21:51–58. https://doi.org/10.1007/s10048-019-00595-0
Zhang M, Windheim M, Roe SM, Peggie M, Cohen P, Prodromou C, Pearl LH (2005) Chaperoned ubiquitylation--crystal structures of the CHIP U box E3 ubiquitin ligase and a CHIP-Ubc13-Uev1a complex. Mol Cell 20(4):525–538. https://doi.org/10.1016/j.molcel.2005.09.023
Harper L, Barkhof F, Fox NC, Schott JM (2015) Using visual rating to diagnose dementia: a critical evaluation of MRI atrophy scales. J Neurol Neurosurg Psychiatry 86(11):1225–1233. https://doi.org/10.1136/jnnp-2014-310090
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol 149(2):351–356. https://doi.org/10.2214/ajr.149.2.351
Bauer P, Stevanin G, Beetz C, Synofzik M, Schmitz-Hubsch T, Wullner U, Berthier E, Ollagnon-Roman E, Riess O, Forlani S, Mundwiller E, Durr A, Schols L, Brice A (2010) Spinocerebellar ataxia type 11 (SCA11) is an uncommon cause of dominant ataxia among French and German kindreds. J Neurol Neurosurg Psychiatry 81(11):1229–1232. https://doi.org/10.1136/jnnp.2009.202150
Synofzik M, Beetz C, Bauer C, Bonin M, Sanchez-Ferrero E, Schmitz-Hubsch T, Wullner U, Nagele T, Riess O, Schols L, Bauer P (2011) Spinocerebellar ataxia type 15: diagnostic assessment, frequency, and phenotypic features. J Med Genet 48(6):407–412. https://doi.org/10.1136/jmg.2010.087023
Wedding IM, Koht J, Dietrichs E, Landro NI, Tallaksen CM (2013) Cognition is only minimally impaired in Spinocerebellar ataxia type 14 (SCA14): a neuropsychological study of ten Norwegian subjects compared to intrafamilial controls and population norm. BMC Neurol 13:186. https://doi.org/10.1186/1471-2377-13-186
Miyoshi Y, Yamada T, Tanimura M, Taniwaki T, Arakawa K, Ohyagi Y, Furuya H, Yamamoto K, Sakai K, Sasazuki T, Kira J (2001) A novel autosomal dominant spinocerebellar ataxia (SCA16) linked to chromosome 8q22.1-24.1. Neurology 57(1):96–100. https://doi.org/10.1212/wnl.57.1.96
Nagaoka U, Takashima M, Ishikawa K, Yoshizawa K, Yoshizawa T, Ishikawa M, Yamawaki T, Shoji S, Mizusawa H (2000) A gene on SCA4 locus causes dominantly inherited pure cerebellar ataxia. Neurology 54(10):1971–1975. https://doi.org/10.1212/wnl.54.10.1971
Stevanin G, Herman A, Brice A, Durr A (1999) Clinical and MRI findings in spinocerebellar ataxia type 5. Neurology 53(6):1355–1357. https://doi.org/10.1212/wnl.53.6.1355
Dohlinger S, Hauser TK, Borkert J, Luft AR, Schulz JB (2008) Magnetic resonance imaging in spinocerebellar ataxias. Cerebellum 7(2):204–214. https://doi.org/10.1007/s12311-008-0025-0
Storey E, Gardner RJ (2012) Spinocerebellar ataxia type 20. Handb Clin Neurol 103:567–573. https://doi.org/10.1016/B978-0-444-51892-7.00038-3
Li X, Zhou C, Cui L, Zhu L, Du H, Liu J, Wang C, Fang S (2018) A case of a novel CACNA1G mutation from a Chinese family with SCA42: a case report and literature review. Medicine (Baltimore) 97(36):e12148. https://doi.org/10.1097/MD.0000000000012148
Nibbeling EAR, Duarri A, Verschuuren-Bemelmans CC, Fokkens MR, Karjalainen JM, Smeets C, de Boer-Bergsma JJ, van der Vries G, Dooijes D, Bampi GB, van Diemen C, Brunt E, Ippel E, Kremer B, Vlak M, Adir N, Wijmenga C, van de Warrenburg BPC, Franke L, Sinke RJ, Verbeek DS (2017) Exome sequencing and network analysis identifies shared mechanisms underlying spinocerebellar ataxia. Brain 140(11):2860–2878. https://doi.org/10.1093/brain/awx251
Ronsin S, Hannoun S, Thobois S, Petiot P, Vighetto A, Cotton F, Tilikete C (2019) A new MRI marker of ataxia with oculomotor apraxia. Eur J Radiol 110:187–192. https://doi.org/10.1016/j.ejrad.2018.11.035
Mol MO, van Rooij JGJ, Brusse E, Verkerk AJMH, Melhem S, den Dunnen WFA, Rizzu P, Cupidi C, van Swieten JC, Donker Kaat L (2020) Clinical and pathologic phenotype of a large family with heterozygous <em>STUB1</em> mutation. Neurology Genetics 6(3):e417. https://doi.org/10.1212/nxg.0000000000000417
Rao SS, Adlard PA (2018) Untangling tau and iron: exploring the interaction between iron and tau in neurodegeneration. Front Mol Neurosci 11:276. https://doi.org/10.3389/fnmol.2018.00276
Inglese M, Petracca M (2013) Imaging multiple sclerosis and other neurodegenerative diseases. Prion 7(1):47–54. https://doi.org/10.4161/pri.22650
Monti S, Borrelli P, Tedeschi E, Cocozza S, Palma G (2017) RESUME: turning an SWI acquisition into a fast qMRI protocol. PLoS One 12(12):e0189933. https://doi.org/10.1371/journal.pone.0189933
Heidelberg D, Ronsin S, Bonneville F, Hannoun S, Tilikete C, Cotton F (2018) Main inherited neurodegenerative cerebellar ataxias, how to recognize them using magnetic resonance imaging? J Neuroradiol 45(5):265–275. https://doi.org/10.1016/j.neurad.2018.05.005
Hewamadduma CA, Hoggard N, O'Malley R, Robinson MK, Beauchamp NJ, Segamogaite R, Martindale J, Rodgers T, Rao G, Sarrigiannis P, Shanmugarajah P, Zis P, Sharrack B, McDermott CJ, Shaw PJ, Hadjivassiliou M (2018) Novel genotype-phenotype and MRI correlations in a large cohort of patients with SPG7 mutations. Neurol Genet 4(6):e279. https://doi.org/10.1212/NXG.0000000000000279
Sanchez-Ferrero E, Coto E, Beetz C, Gamez J, Corao AI, Diaz M, Esteban J, del Castillo E, Moris G, Infante J, Menendez M, Pascual-Pascual SI, Lopez de Munain A, Garcia-Barcina MJ, Alvarez V (2013) SPG7 mutational screening in spastic paraplegia patients supports a dominant effect for some mutations and a pathogenic role for p.A510V. Clin Genet 83(3):257–262. https://doi.org/10.1111/j.1399-0004.2012.01896.x
Coutinho P, Barbot C (1993) Ataxia with oculomotor apraxia type 1. doi:NBK1456 [bookaccession]
Moreira MC, Koenig M (1993) Ataxia with oculomotor apraxia type 2. doi:NBK1154 [bookaccession]
Bond KM, Brinjikji W, Eckel LJ, Kallmes DF, McDonald RJ, Carr CM (2017) Dentate update: imaging features of entities that affect the dentate nucleus. AJNR Am J Neuroradiol 38(8):1467–1474. https://doi.org/10.3174/ajnr.A5138
Khadilkar S, Jaggi S, Patel B, Yadav R, Hanagandi P, Faria do Amaral LL (2016) A practical approach to diseases affecting dentate nuclei. Clin Radiol 71(1):107–119. https://doi.org/10.1016/j.crad.2015.09.010
Abdelhalim AN, Alberico RA, Barczykowski AL, Duffner PK (2014) Patterns of magnetic resonance imaging abnormalities in symptomatic patients with Krabbe disease correspond to phenotype. Pediatr Neurol 50(2):127–134. https://doi.org/10.1016/j.pediatrneurol.2013.10.001
Fourati H, Ellouze E, Ahmadi M, Chaari D, Kamoun F, Hsairi I, Triki C, Mnif Z (2016) MRI features in 17 patients with l2 hydroxyglutaric aciduria. Eur J Radiol Open 3:245–250. https://doi.org/10.1016/j.ejro.2016.09.001
Nunes J, Loureiro S, Carvalho S, Pais RP, Alfaiate C, Faria A, Garcia P, Diogo L (2013) Brain MRI findings as an important diagnostic clue in glutaric aciduria type 1. Neuroradiol J 26(2):155–161. https://doi.org/10.1177/197140091302600204
van der Knaap MS, Naidu S, Breiter SN, Blaser S, Stroink H, Springer S, Begeer JC, van Coster R, Barth PG, Thomas NH, Valk J, Powers JM (2001) Alexander disease: diagnosis with MR imaging. AJNR Am J Neuroradiol 22(3):541–552
Yalcinkaya C, Benbir G, Salomons GS, Karaarslan E, Rolland MO, Jakobs C, van der Knaap MS (2005) Atypical MRI findings in Canavan disease: a patient with a mild course. Neuropediatrics 36(5):336–339. https://doi.org/10.1055/s-2005-872878
De Stefano N, Dotti MT, Mortilla M, Federico A (2001) Magnetic resonance imaging and spectroscopic changes in brains of patients with cerebrotendinous xanthomatosis. Brain 124(Pt 1):121–131. https://doi.org/10.1093/brain/124.1.121
Zuccoli G, Santa Cruz D, Bertolini M, Rovira A, Gallucci M, Carollo C, Pipitone N (2009) MR imaging findings in 56 patients with Wernicke encephalopathy: nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol 30(1):171–176. https://doi.org/10.3174/ajnr.A1280
Lasek K, Lencer R, Gaser C, Hagenah J, Walter U, Wolters A, Kock N, Steinlechner S, Nagel M, Zuhlke C, Nitschke MF, Brockmann K, Klein C, Rolfs A, Binkofski F (2006) Morphological basis for the spectrum of clinical deficits in spinocerebellar ataxia 17 (SCA17). Brain 129(Pt 9):2341–2352. https://doi.org/10.1093/brain/awl148
Acknowledgements
The authors would like to thank Giuseppe De Michele, MD, Alessandro Filla, MD, Filippo Maria Santorelli, MD, PhD, and Gabriella Silvestri, MD, PhD, for all the insightful suggestions provided during the preparation of this manuscript.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S.C. received fees for speaking from Genzyme and Shire, fees for adv. board from Amicus. All other authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Cocozza, S., Pontillo, G., De Michele, G. et al. The “crab sign”: an imaging feature of spinocerebellar ataxia type 48. Neuroradiology 62, 1095–1103 (2020). https://doi.org/10.1007/s00234-020-02427-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02427-7