Abstract
Purpose
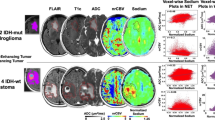
Recent advances in sodium brain MRI have allowed for increased signal-to-noise ratio, faster imaging, and the ability of differentiating intracellular from extracellular sodium concentration, opening a new window of opportunity for clinical application. In gliomas, there are significant alterations in sodium metabolism, including increase in the total sodium concentration and extracellular volume fraction. The purpose of this study is to assess the feasibility of using sodium MRI quantitative measurements to evaluate gliomas.
Methods
Eight patients with treatment-naïve gliomas were scanned at 3 T with a homemade 1H/23Na head coil, generating maps of pseudo-intracellular sodium concentration (C1), pseudo-extracellular volume fraction (α2), apparent intracellular sodium concentration (aISC), and apparent total sodium concentration (aTSC). Measurements were made within the contralateral normal-appearing putamen, contralateral normal-appearing white matter (NAWM), and solid tumor regions (area of T2-FLAIR abnormality, excluding highly likely areas of edema, cysts, or necrosis). Paired samples t test were performed comparing NAWM and putamen and between NAWM and solid tumor.
Results
The normal-appearing putamen demonstrated significantly higher values for aTSC, aISC, C1 (p < 0.001), and α2 (p = 0.002) when compared to those of NAWM. The mean average of all solid tumors, when compared to that of NAWM, demonstrated significantly higher values of aTSC and α2 (p < 0.001), and significantly lower values of aISC (p = 0.02) for each patient. There was no significant difference between the values of C1 (p = 0.19).
Conclusion
Quantitative sodium measurements can be done in glioma patients and also has provided further evidence that total sodium and extracellular volume fraction are increased in gliomas.




Similar content being viewed by others
References
Ouwerkerk R (2011) Sodium MRI. In: Modo M, Bulte J (eds) Magnetic Resonance Neuroimaging. Humana Press, New York, pp 175–201
Maeno E, Takahashi N, Okada Y (2006) Dysfunction of regulatory volume increase is a key component of apoptosis. FEBS Lett 580:6513–6517
Perman W, Turski P, Houston L et al (1986) Methodology of in vivo human sodium MR imaging at 1.5 T. Radiology 160:811–820
Madelin G, Regatte R (2013) Biomedical applications of sodium MRI in vivo. J Magn Reson Imaging 38:511–529
Turski P, Houston L, Perman W et al (1987) Experimental and human brain neoplasms: detection with in vivo sodium MR imaging. Radiology 163:245–249
Boada F, Gillen J, Shen G et al (1997) Fast three dimensional sodium imaging. Magn Reson Med 37:706–715
Madelin G, Kline R, Walvick R et al (2014) A method for estimating intracellular sodium concentration and extracellular volume fraction in brain in vivo using sodium magnetic resonance imaging. Sci Rep 4:4763
Pipe J, Zwart N, Aboussouan E et al (2011) A new design and rationale for 3D orthogonally oversampled k-space trajectories. Magn Reson Med 66:1303–1311
Boada F, Tanase C, Davis D et al (2004) Non-invasive assessment of tumor proliferation using triple quantum filtered 23/Na MRI: technical challenges and solutions. Conf Proc IEEE Eng Med Biol Soc 7:5238–5241
Ridley B, Nagel AM, Bydder M, Maarouf A, Stellmann JP, Gherib S, Verneuil J, Viout P, Guye M, Ranjeva JP, Zaaraoui W (2018) Distribution of brain sodium long and short relaxation times and concentrations: a multi-echo ultra-high field (23)Na MRI study. Sci Rep 8:4357
Cameron I, Smith N, Pool T et al (1980) Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res 40:1439–14500
Cong D, Zhu W, Kuo J et al (2015) Ion transporters in brain tumors. Curr Med Chem 22:1171–1181
Madelin G, Babb J, Xia D, Regatte RR (2015) Repeatability of quantitative sodium magnetic resonance imaging for estimating pseudo-intracellular sodium concentration and pseudo-extracellular volume fraction in brain at 3 T. PLoS One 10:e0118692
Bartha R, Megyesi J, Watling C (2008) Low-grade glioma: correlation of short echo time 1H-MR spectroscopy with 23Na MR imaging. AJNR Am J Neuroradiol 29:464–470
Ouwerkerk R, Bleich K, Gillen J et al (2003) Tissue sodium concentration in human brain tumors as measured with 23Na MR imaging. Radiology 227:529–537
Nagel A, Bock M, Hartmann C et al (2011) The potential of relaxation-weighted sodium magnetic resonance imaging as demonstrated on brain tumors. Investig Radiol 46:539–547
Ridley B, Marchi A, Wirsich J, Soulier E, Confort-Gouny S, Schad L, Bartolomei F, Ranjeva JP, Guye M, Zaaraoui W (2017) Brain sodium MRI in human epilepsy: disturbances of ionic homeostasis reflect the organization of pathological regions. Neuroimage 157:173–183
Zamecnik J, Vargova L, Homola A et al (2004) Extracellular matrix glycoproteins and diffusion barriers in human astrocytic tumours. Neuropathol Appl Neurobiol 30:338–350
Zamecnik J (2005) The extracellular space and matrix of gliomas. Acta Neuropathol 110:435–442
Bakay L (1970) The extracellular space in brain tumours: I. Morphological considerations. Brain 93:693–698
Bakay L (1970) The extracellular space in brain tumours: II. The sucrose space. Brain 93:699–708
Vargova L, Homola A, Zamecnik J et al (2003) Diffusion parameters of the extracellular space in human gliomas. Glia 42:77–88
Biller A, Badde S, Nagel A, Neumann JO, Wick W, Hertenstein A, Bendszus M, Sahm F, Benkhedah N, Kleesiek J (2016) Improved brain tumor classification by sodium MR imaging: prediction of IDH mutation status and tumor progression. AJNR Am J Neuroradiol 37:66–73
Cong D, Zhu W, Shi Y, Pointer KB, Clark PA, Shen H, Kuo JS, Hu S, Sun D (2014) Upregulation of NHE1 protein expression enables glioblastoma cells to escape TMZ-mediated toxicity via increased H(+) extrusion, cell migration and survival. Carcinogenesis 35:2014–2024
Zhu W, Carney K, Pigott V et al (2016) Glioma-mediated microglial activation promotes glioma proliferation and migration: roles of Na+/H+ exchanger isoform 1. Carcinogenesis 37:839–851
Blough M, Al-Najjar M, Chesnelong C et al (2012) DNA hypermethylation and 1p loss silence NHE-1 in oligodendroglioma. Ann Neurol 71:845–849
Koessler L, Colnat-Coulbois S, Cecchin T, Hofmanis J, Dmochowski JP, Norcia AM, Maillard LG (2017) In-vivo measurements of human brain tissue conductivity using focal electrical current injection through intracerebral multicontact electrodes. Hum Brain Mapp 38:974–986
Gelman N, Ewing JR, Gorell JM, Spickler EM, Solomon EG (2001) Interregional variation of longitudinal relaxation rates in human brain at 3.0 T: relation to estimated iron and water contents. Magn Reson Med 45:71–79
Krebs N, Langkammer C, Goessler W, Ropele S, Fazekas F, Yen K, Scheurer E (2014) Assessment of trace elements in human brain using inductively coupled plasma mass spectrometry. J Trace Elem Med Biol 28:1–7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded in part by the National Institutes of Health (NIH): 1R03AR065763 (GM), 1R01NS097494 (GM) and 1R21CA213169 (GM), and the NIBIB for the P41 (CAI2R), a NIBIB Biomedical Technology Resource Center (NIH: 1P41EB017183) program grant.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
This study was presented as an oral presentation at the American Society of Neuroradiology (ASNR) 55th Annual Meeting, April 24–27, 2017, Long Beach, CA, USA.
Rights and permissions
About this article
Cite this article
Nunes Neto, L.P., Madelin, G., Sood, T.P. et al. Quantitative sodium imaging and gliomas: a feasibility study. Neuroradiology 60, 795–802 (2018). https://doi.org/10.1007/s00234-018-2041-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-018-2041-1