Abstract
Purpose
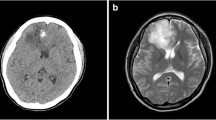
Imaging findings of pilocytic astrocytoma (PA) vary widely, sometimes resembling those of high-grade glioma (HGG). This study aimed to identify the imaging parameters that can be used to differentiate PA from HGG.
Methods
Altogether, 60 patients with PAs and 138 patients with HGGs were included in the study. Tumor properties and the presence of hydrocephalus, peritumoral edema, and dissemination were evaluated. We also measured the maximum relative cerebral blood flow (rCBFmax) and volume (rCBVmax) and determined the minimum apparent diffusion coefficient (ADCmin) in the tumor’s solid components. The relative T1 (rT1), T2 (rT2), and contrast-enhanced T1 (rCE-T1) intensity values were evaluated. Parameters were compared between PAs and HGGs using the Mann–Whitney U test. Receiver operating characteristic (ROC) curve analysis was also used to evaluate these imaging parameters. A value of P < .05 was considered to indicate significance.
Results
Intratumoral hemorrhage and calcification were observed in 10.0% and 21.7% of PAs, respectively. The rCBFmax and rCBVmax values were significantly lower in PAs (0.50 ± 0.35, 1.82 ± 1.21) than those in HGGs (2.98 ± 1.80, 9.54 ± 6.88) (P < .0001, P = .0002, respectively). The ADCmin values were significantly higher in PAs (1.36 ± 0.56 × 10−3 mm2/s) than those in HGGs (0.86 ± 0.37 × 10−3 mm2/s) (P < .0001). ROC analysis showed that the best diagnostic performance was achieved with rCBFmax.
Conclusion
The rCBFmax, rCBVmax, and ADCmin can differentiate PAs from HGGs.



Similar content being viewed by others
References
Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2015) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro-Oncology 17(Suppl 4):iv1–iv62. https://doi.org/10.1093/neuonc/nov189
Louis D, Ohgaki H, Wiestler O, Cavenee W, Ellison D, Figarella-Branger D, Perry A, Reifenberger G, von Deimling A (2016) WHO classification of tumours of the central nervous system, revised. IARC Press, Lyon
Bell D, Chitnavis BP, Al-Sarraj S, Connor S, Sharr MM, Gullan RW (2004) Pilocytic astrocytoma of the adult—clinical features, radiological features and management. Br J Neurosurg 18(6):613–616
Murakami R, Hirai T, Kitajima M, Fukuoka H, Toya R, Nakamura H, Kuratsu J, Yamashita Y (2008) Magnetic resonance imaging of pilocytic astrocytomas: usefulness of the minimum apparent diffusion coefficient (ADC) value for differentiation from high-grade gliomas. Acta Radiol 49(4):462–467. https://doi.org/10.1080/02841850801918555
Koeller KK, Rushing EJ (2004) From the archives of the AFIP: pilocytic astrocytoma: radiologic-pathologic correlation. Radiographics 24(6):1693–1708. https://doi.org/10.1148/rg.246045146
Fulham MJ, Melisi JW, Nishimiya J, Dwyer AJ, Di Chiro G (1993) Neuroimaging of juvenile pilocytic astrocytomas: an enigma. Radiology 189(1):221–225. https://doi.org/10.1148/radiology.189.1.8372197
Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY (2003) Primary brain tumours in adults. Lancet 361(9354):323–331. https://doi.org/10.1016/S0140-6736(03)12328-8
Arai K, Sato N, Aoki J, Yagi A, Taketomi-Takahashi A, Morita H, Koyama Y, Oba H, Ishiuchi S, Saito N, Endo K (2006) MR signal of the solid portion of pilocytic astrocytoma on T2-weighted images: is it useful for differentiation from medulloblastoma? Neuroradiology 48(4):233–237. https://doi.org/10.1007/s00234-006-0048-5
Togao O, Hiwatashi A, Yamashita K, Kikuchi K, Mizoguchi M, Yoshimoto K, Suzuki SO, Iwaki T, Obara M, Van Cauteren M, Honda H (2016) Differentiation of high-grade and low-grade diffuse gliomas by intravoxel incoherent motion MR imaging. Neuro-Oncology 18(1):132–141. https://doi.org/10.1093/neuonc/nov147
Sugahara T, Korogi Y, Kochi M, Ikushima I, Hirai T, Okuda T, Shigematsu Y, Liang L, Ge Y, Ushio Y, Takahashi M (1998) Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol 171(6):1479–1486. https://doi.org/10.2214/ajr.171.6.9843274
de Fatima Vasco Aragao M, Law M, Batista de Almeida D, Fatterpekar G, Delman B, Bader AS, Pelaez M, Fowkes M, Vieira de Mello R, Moraes Valenca M (2014) Comparison of perfusion, diffusion, and MR spectroscopy between low-grade enhancing pilocytic astrocytomas and high-grade astrocytomas. AJNR Am J Neuroradiol 35(8):1495–1502. https://doi.org/10.3174/ajnr.A3905
Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, Lu H, MacIntosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73(1):102–116. https://doi.org/10.1002/mrm.25197
Bulakbasi N, Kocaoglu M, Farzaliyev A, Tayfun C, Ucoz T, Somuncu I (2005) Assessment of diagnostic accuracy of perfusion MR imaging in primary and metastatic solitary malignant brain tumors. AJNR Am J Neuroradiol 26(9):2187–2199
Grand SD, Kremer S, Tropres IM, Hoffmann DM, Chabardes SJ, Lefournier V, Berger FR, Pasteris C, Krainik A, Pasquier BM, Peoch M, Le Bas JF (2007) Perfusion-sensitive MRI of pilocytic astrocytomas: initial results. Neuroradiology 49(7):545–550. https://doi.org/10.1007/s00234-006-0204-y
Yeom KW, Mitchell LA, Lober RM, Barnes PD, Vogel H, Fisher PG, Edwards MS (2014) Arterial spin-labeled perfusion of pediatric brain tumors. AJNR Am J Neuroradiol 35(2):395–401. https://doi.org/10.3174/ajnr.A3670
Bull JG, Saunders DE, Clark CA (2012) Discrimination of paediatric brain tumours using apparent diffusion coefficient histograms. Eur Radiol 22(2):447–457. https://doi.org/10.1007/s00330-011-2255-7
Shibahara I, Kanamori M, Kumabe T, Endo H, Sonoda Y, Ogawa Y, Watanabe M, Tominaga T (2009) Hemorrhagic onset of pilocytic astrocytoma and pilomyxoid astrocytoma. Brain Tumor Pathol 26(1):1–5. https://doi.org/10.1007/s10014-008-0243-7
Coakley KJ, Huston J 3rd, Scheithauer BW, Forbes G, Kelly PJ (1995) Pilocytic astrocytomas: well-demarcated magnetic resonance appearance despite frequent infiltration histologically. Mayo Clin Proc 70(8):747–751. https://doi.org/10.1016/S0025-6196(11)64346-2
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109. https://doi.org/10.1007/s00401-007-0243-4
Henson JW, Gaviani P, Gonzalez RG (2005) MRI in treatment of adult gliomas. Lancet Oncol 6(3):167–175. https://doi.org/10.1016/S1470-2045(05)01767-5
Pencalet P, Maixner W, Sainte-Rose C, Lellouch-Tubiana A, Cinalli G, Zerah M, Pierre-Kahn A, Hoppe-Hirsch E, Bourgeois M, Renier D (1999) Benign cerebellar astrocytomas in children. J Neurosurg 90(2):265–273. https://doi.org/10.3171/jns.1999.90.2.0265
Cha S, Knopp EA, Johnson G, Wetzel SG, Litt AW, Zagzag D (2002) Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology 223(1):11–29. https://doi.org/10.1148/radiol.2231010594
Nelson SJ, Cha S (2003) Imaging glioblastoma multiforme. Cancer J 9(2):134–145
Martin AJ, Liu H, Hall WA, Truwit CL (2001) Preliminary assessment of turbo spectroscopic imaging for targeting in brain biopsy. AJNR Am J Neuroradiol 22(5):959–968
Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24(10):1989–1998
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Bayer Yakuhin Ltd. The study data were independently analyzed and interpreted by the funder.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
For this type of retrospective study formal consent is not required.
Electronic supplementary material
Supplementary Figure S1
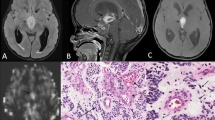
Placement of regions of interests (ROIs). Neuroradiologists in each institution independently placed three circular ROIs (circles, at left) in the solid component of a tumor. Cystic, necrotic, or hemorrhagic components were avoided with reference to conventional magnetic resonance imaging. An ROI (single circle, at right) was also placed in the contralateral normal-appearing gray matter. (GIF 402 kb)
Supplementary Figure S2
ROC analysis of all pilocytic astrocytomas and high-grade gliomas. ROC analysis shows the best diagnostic performance with rCBFmax. Sensitivity, specificity, and area under the curve are 100%, 91.7%, and 0.98 for rCBFmax; 100%, 76.7%, and 0.92 for rCBVmax; 63.3%, 91.3%, and 0.78 for ADCmin; 41.7%, 79.0%, and 0.60 for rT1; 30.0%, 83.3%, and 0.55 for rCE-T1; and 30.0%, 78.3%, and 0.51 for rT2, respectively. ROC receiver operating characteristic, rCBF max maximum relative cerebral blood flow, AUC area under the curve, rCBV max maximum relative cerebral blood flow, ADC min minimum apparent diffusion coefficient, rT1 relative non-contrast T1-weighted imaging, rT2 relative T2-weighted imaging, rCE-T1 relative contrast-enhanced T1-weighted imaging (GIF 8 kb)
Rights and permissions
About this article
Cite this article
Kikuchi, K., Hiwatashi, A., Togao, O. et al. Usefulness of perfusion- and diffusion-weighted imaging to differentiate between pilocytic astrocytomas and high-grade gliomas: a multicenter study in Japan. Neuroradiology 60, 391–401 (2018). https://doi.org/10.1007/s00234-018-1991-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-018-1991-7