Abstract
Purpose
Abnormal brain intrinsic functional connectivity (FC) has been documented in minimal hepatic encephalopathy (MHE) by static connectivity analysis. However, changes in dynamic FC (dFC) remain unknown. We aimed to identify altered dFC within the default mode network (DMN) associated with MHE.
Methods
Resting-state functional MRI data were acquired from 20 cirrhotic patients with MHE and 24 healthy controls. DMN seed regions were defined using seed-based FC analysis (centered on the posterior cingulate cortex (PCC)). Dynamic FC architecture was calculated using a sliding time-window method. K-means clustering (number of clusters = 2–4) was applied to estimate FC states.
Results
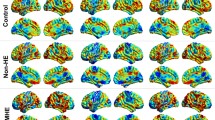
When the number of clusters was 2, MHE patients presented weaker connectivity strengths compared with controls in states 1 and 2. In state 1, decreased FC strength was found between the PCC/precuneus (PCUN) and right medial temporal lobe (MTL)/bilateral lateral temporal cortex (LTC); left inferior parietal lobule (IPL) and right MTL/left LTC; right IPL and right MTL/bilateral LTC; right MTL and right LTC; and medial prefrontal cortex (MPFC) and right MTL/bilateral LTC. In state 2, reduced FC strength was observed between the PCC/PCUN and bilateral MTL/bilateral LTC; left IPL and left MTL/bilateral LTC/MPFC; and left LTC and right LTC. Altered connectivities from state 1 were correlated with patient cognitive performance. Similar findings were observed when the number of clusters was set to 3 or 4.
Conclusion
Aberrant dynamic DMN connectivity is an additional characteristic of MHE. Dynamic connectivity analysis offers a novel paradigm for understanding MHE-related mechanisms.





Similar content being viewed by others
Abbreviations
- MHE:
-
minimal hepatic encephalopathy
- HE:
-
hepatic encephalopathy
- FC:
-
functional connectivity
- DMN:
-
default mode network
- dFC:
-
dynamic functional connectivity
- HCs:
-
healthy controls
- PHES:
-
psychometric hepatic encephalopathy score
- MNI:
-
Montreal Neurological Institute
- PCC:
-
posterior cingulate cortex
- FWE:
-
family-wise error
- ROI:
-
region of interest
- PCC/PCUN:
-
posterior cingulate cortex/precuneus
- MPFC:
-
medial prefrontal cortex
- IPL:
-
inferior parietal lobule
- MTL:
-
medial temporal lobe
- LTC:
-
lateral temporal cortex
- ICA:
-
independent component analysis
References
Bajaj JS, Wade JB, Sanyal AJ (2009) Spectrum of neurocognitive impairment in cirrhosis: implications for the assessment of hepatic encephalopathy. Hepatology 50:2014–2021
Romero-Gomez M, Boza F, Garcia-Valdecasas MS, Garcia E, Aguilar-Reina J (2001) Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol 96:2718–2723
Groeneweg M, Quero JC, De Bruijn I et al (1998) Subclinical hepatic encephalopathy impairs daily functioning. Hepatology 28:45–49
Dhiman RK, Kurmi R, Thumburu KK et al (2010) Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Dig Dis Sci 55:2381–2390
Chen HJ, Jiao Y, Zhu XQ et al (2013) Brain dysfunction primarily related to previous overt hepatic encephalopathy compared with minimal hepatic encephalopathy: resting-state functional MR imaging demonstration. Radiology 266:261–270
Qi R, Zhang LJ, Xu Q et al (2014) Abnormal functional connectivity within the default mode network in patients with HBV-related cirrhosis without hepatic encephalopathy revealed by resting-state functional MRI. Brain Res 1576:73–80
Qi R, Zhang LJ, Xu Q et al (2012) Selective impairments of resting-state networks in minimal hepatic encephalopathy. PLoS One 7:e37400
Hsu TW, Wu CW, Cheng YF et al (2012) Impaired small-world network efficiency and dynamic functional distribution in patients with cirrhosis. PLoS One 7:e35266
Hutchison RM, Womelsdorf T, Allen EA et al (2013) Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage 80:360–378
Calhoun VD, Miller R, Pearlson G, Adali T (2014) The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron 84:262–274
Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014) Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676
Jones DT, Vemuri P, Murphy MC et al (2012) Non-stationarity in the "resting brain's" modular architecture. PLoS One 7:e39731
Rashid B, Damaraju E, Pearlson GD, Calhoun VD (2014) Dynamic connectivity states estimated from resting fMRI identify differences among schizophrenia, bipolar disorder, and healthy control subjects. Front Hum Neurosci 8:897
Liao W, Zhang Z, Mantini D et al (2014) Dynamical intrinsic functional architecture of the brain during absence seizures. Brain Struct Funct 219:2001–2015
Chen HJ, Jiang LF, Sun T, Liu J, Chen QF, Shi HB (2015) Resting-state functional connectivity abnormalities correlate with psychometric hepatic encephalopathy score in cirrhosis. Eur J Radiol 84:2287–2295
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38
Li X, Zhu D, Jiang X et al (2014) Dynamic functional connectomics signatures for characterization and differentiation of PTSD patients. Hum Brain Mapp 35:1761–1778
Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012) Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 22:158–165
Yu Q, Erhardt EB, Sui J et al (2015) Assessing dynamic brain graphs of time-varying connectivity in fMRI data: application to healthy controls and patients with schizophrenia. NeuroImage 107:345–355
Zalesky A, Breakspear M (2015) Towards a statistical test for functional connectivity dynamics. NeuroImage 114:466–470
Leonardi N, Van De Ville D (2015) On spurious and real fluctuations of dynamic functional connectivity during rest. NeuroImage 104:430–436
Damaraju E, Allen EA, Belger A et al (2014) Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin 5:298–308
Du Y, Pearlson GD, Yu Q et al (2016) Interaction among subsystems within default mode network diminished in schizophrenia patients: a dynamic connectivity approach. Schizophr Res 170:55–65
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678
Lin WC, Hsu TW, Chen CL et al (2012) Connectivity of default-mode network is associated with cerebral edema in hepatic encephalopathy. PLoS One 7:e36986
Qi R, Zhang LJ, Luo S et al (2014) Default mode network functional connectivity: a promising biomarker for diagnosing minimal hepatic encephalopathy: CONSORT-compliant article. Medicine (Baltimore) 93:e227
Iversen P, Sorensen M, Bak LK et al (2009) Low cerebral oxygen consumption and blood flow in patients with cirrhosis and an acute episode of hepatic encephalopathy. Gastroenterology 136:863–871
Chen HJ, Zhu XQ, Shu H et al (2012) Structural and functional cerebral impairments in cirrhotic patients with a history of overt hepatic encephalopathy. Eur J Radiol 81:2463–2469
Guevara M, Baccaro ME, Gomez-Anson B et al (2011) Cerebral magnetic resonance imaging reveals marked abnormalities of brain tissue density in patients with cirrhosis without overt hepatic encephalopathy. J Hepatol 55:564–573
Shakil S, Lee CH, Keilholz SD (2016) Evaluation of sliding window correlation performance for characterizing dynamic functional connectivity and brain states. NeuroImage 133:111–128
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was funded by a grant from the National Natural Science Foundation of China (No. 81501450) and a project funded by the China Postdoctoral Science Foundation (No. 2015M580452).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the Medical Research Ethics Committee of Fujian Medical University Union Hospital and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Supplementary Figure 1
The common states (matrix) of functional connectivity within the DMN, which was extracted using the K-means clustering method (with the number of clusters = 3 and the window size = 40s) and the visualized network pattern of the common functional connectivity states at a threshold of 0.35. The line sizes indicate functional connectivity strength in the states. (GIF 1526 kb)
Supplementary Figure 2
The common states (matrix) of functional connectivity within the DMN, which was extracted using the K-means clustering method (with the number of clusters = 4 and the window size = 40s) and the visualized network pattern of the common functional connectivity states at a threshold of 0.35. The line sizes indicate functional connectivity strength in the states. (GIF 2078 kb)
Supplementary Figure 3
The two-sample t-tests results from comparing subject-specific states between the 2 groups (with the number of clusters = 3 and the window size = 40s) and the visualized aberrant connectivities for states 1, 2, and 3. The line sizes indicate significance of between-group differences in functional connectivity. A significant reduction of functional connectivity was found during states 1, 2, and 3, while no increased connectivity was observed in MHE patients. The “*” denotes significantly decreased connectivity (P < 0.05, uncorrected) in the patient group. (GIF 1520 kb)
Supplementary Figure 4
The two-sample t-tests results from comparing subject-specific states between the 2 groups (with the number of clusters = 4 and the window size = 40s) and the visualized aberrant connectivities for states 1, 2, 3, and 4. The line sizes indicate significance of between-group differences in functional connectivity. A significant reduction of functional connectivity was found during states 1, 2, 3, and 4, while no increased connectivity was observed in MHE patients. The “*” denotes significantly decreased connectivity (P < 0.05, uncorrected) in the patient group. (GIF 1998 kb)
Supplementary Figure 5
The common states of functional connectivity within the DMN, which were extracted using the K-means clustering method (with the number of clusters = 2 and the window size = 100 s) and the visualized network pattern of the common functional connectivity states at a threshold of 0.35. The line sizes indicate functional connectivity strength in the states. (GIF 365 kb)
Supplementary Figure 6
The two-sample t-tests results from comparing subject-specific states between the 2 groups (with the number of clusters = 2 and the window size = 100 s) and the visualized aberrant connectivities for states 1 and 2. The line sizes indicate significance of between-group differences in functional connectivity. A significant reduction of functional connectivity was found during states 1 and 2 (P < 0.05, uncorrected), while no increased connectivity was observed in MHE patients. (GIF 365 kb)
Rights and permissions
About this article
Cite this article
Chen, HJ., Lin, HL., Chen, QF. et al. Altered dynamic functional connectivity in the default mode network in patients with cirrhosis and minimal hepatic encephalopathy. Neuroradiology 59, 905–914 (2017). https://doi.org/10.1007/s00234-017-1881-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1881-4