Abstract
Purpose
Brain connectivity is highly dynamic, but functional connectivity (FC) studies using resting-state functional magnetic resonance imaging (rs-fMRI) assume it to be static. This study assessed differences in dynamic FC between young healthy adults (YH) and elderly healthy adults (EH) compared to static FC.
Methods
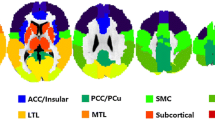
Using rs-fMRI data from 12 YH and 31 EH, FC was assessed in six functional regions (subcortical, auditory [AUD], sensorimotor [SM], visuospatial [VS], cognitive control [CC], and default mode network [DMN]). Static FC was calculated as Fisher’s z-transformed correlation coefficient. The sliding time window correlation (window size 30 s, step size 3 s) was applied for dynamic FC, and the standard deviation across sliding windows was calculated. Differences in static and dynamic FC between EH and YH were calculated and compared by region.
Results
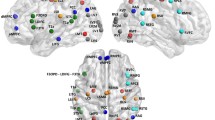
EH showed decreased static FC in the subcortical, CC, and DMN regions (FDR corrected p = 0.0013; 74 regions), with no regions showing static FC higher than that in YH. EH showed increased dynamic FC in the subcortical, CC, and DMN regions, whereas decreased dynamic FC in CC and DMN regions (p < 0.01). However, the regions showing differences between EH and YH did not overlap between static and dynamic FC.
Conclusion
Dynamic FC exhibited differences from static FC in EH and YH, mainly in regions involved in cognitive control and the DMN. Altered dynamic FC demonstrated both qualitatively and quantitatively distinct patterns of transient brain activity and needs to be studied as an imaging biomarker in the aging process.




Similar content being viewed by others
References
Kenny ER, Blamire AM, Firbank MJ, O’Brien JT (2012) Functional connectivity in cortical regions in dementia with Lewy bodies and Alzheimer’s disease. Brain 135:569–581. doi:10.1093/brain/awr327
Greicius MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 101:4637–4642. doi:10.1073/pnas.0308627101
Voets NL, Beckmann CF, Cole DM, Hong S, Bernasconi A, Bernasconi N (2012) Structural substrates for resting network disruption in temporal lobe epilepsy. Brain 135:2350–2357. doi:10.1093/brain/aws137
Shinotoh H, Tessitore A (2014) Resting-state fMRI sheds light on neural substrates of cognitive decline in Parkinson disease. Neurology 83:2000–2001. doi:10.1212/WNL.0000000000001037
Gomez-Ramirez J, Li Y, Wu Q, Wu J (2015) A quantitative study of network robustness in resting-state fMRI in young and elder adults. Front Aging Neurosci 7:256. doi:10.3389/fnagi.2015.00256
Ward AM, Mormino EC, Huijbers W, Schultz AP, Hedden T, Sperling RA (2015) Relationships between default-mode network connectivity, medial temporal lobe structure, and age-related memory deficits. Neurobiol Aging 36:265–272. doi:10.1016/j.neurobiolaging.2014.06.028
Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S (2015) Signature of consciousness in the dynamics of resting-state brain activity. Proc Natl Acad Sci U S A 112:887–892. doi:10.1073/pnas.1418031112
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678. doi:10.1073/pnas.0504136102
de Pasquale F, Della Penna S, Snyder AZ et al (2010) Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci U S A 107:6040–6045. doi:10.1073/pnas.0913863107
Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014) Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676. doi:10.1093/cercor/bhs352
Marusak HA, Calhoun VD, Brown S et al (2016) Dynamic functional connectivity of neurocognitive networks in children. Hum Brain Mapp. doi:10.1002/hbm.23346
Damaraju E, Allen EA, Belger A et al (2014) Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuro Image Clin 5:298–308. doi:10.1016/j.nicl.2014.07.003
Liu F, Wang Y, Li M et al (2016) Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic-clonic seizure. Hum Brain Mapp. doi:10.1002/hbm.23430
Campbell KL, Grigg O, Saverino C, Churchill N, Grady CL (2013) Age differences in the intrinsic functional connectivity of default network subsystems. Front Aging Neurosci 5:73. doi:10.3389/fnagi.2013.00073
Sambataro F, Murty VP, Callicott JH et al (2010) Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging 31:839–852. doi:10.1016/j.neurobiolaging.2008.05.022
Andrews-Hanna JR, Snyder AZ, Vincent JL et al (2007) Disruption of large-scale brain systems in advanced aging. Neuron 56:924–935. doi:10.1016/j.neuron.2007.10.038
Mevel K, Chetelat G, Eustache F, Desgranges B (2011) The default mode network in healthy aging and Alzheimer’s disease. Int J Alzheimers Dis 2011:535816. doi:10.4061/2011/535816
Rajah MN, Bastianetto S, Bromley-Brits K et al (2009) Biological changes associated with healthy versus pathological aging: a symposium review. Ageing Res Rev 8:140–146
Cabeza R, Anderson ND, Locantore JK, McIntosh AR (2002) Aging gracefully: compensatory brain activity in high-performing older adults. Neuro Image 17:1394–1402
Jimura K, Braver TS (2010) Age-related shifts in brain activity dynamics during task switching. Cereb Cortex 20:1420–1431. doi:10.1093/cercor/bhp206
Sala-Llonch R, Bartres-Faz D, Junque C (2015) Reorganization of brain networks in aging: a review of functional connectivity studies. Front Psychol 6:663. doi:10.3389/fpsyg.2015.00663
Johnson R Jr, Nessler D, Friedman D (2013) Temporally specific divided attention tasks in young adults reveal the temporal dynamics of episodic encoding failures in elderly adults. Psychol Aging 28:443–456. doi:10.1037/a0030967
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173
Leonardi N, Van De Ville D (2015) On spurious and real fluctuations of dynamic functional connectivity during rest. Neuro Image 104:430–436. doi:10.1016/j.neuroimage.2014.09.007
Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW (2010) Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuro Image 52:571–582. doi:10.1016/j.neuroimage.2010.04.246
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system : an approach to cerebral imaging. Georg Thieme, Stuttgart ; New York
Xia M, Wang J, He Y (2013) BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One 8:e68910. doi:10.1371/journal.pone.0068910
Damoiseaux JS, Beckmann CF, Arigita EJ et al (2008) Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex 18:1856–1864. doi:10.1093/cercor/bhm207
Hoffstaedter F, Grefkes C, Roski C, Caspers S, Zilles K, Eickhoff SB (2015) Age-related decrease of functional connectivity additional to gray matter atrophy in a network for movement initiation. Brain Struct Funct 220:999–1012. doi:10.1007/s00429-013-0696-2
Demirtas M, Tornador C, Falcon C et al (2016) Dynamic functional connectivity reveals altered variability in functional connectivity among patients with major depressive disorder. Hum Brain Mapp 37:2918–2930. doi:10.1002/hbm.23215
Hindriks R, Adhikari MH, Murayama Y et al (2016) Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI? Neuro Image 127:242–256. doi:10.1016/j.neuroimage.2015.11.055
Cole MW, Pathak S, Schneider W (2010) Identifying the brain’s most globally connected regions. Neuro Image 49:3132–3148. doi:10.1016/j.neuroimage.2009.11.001
Filippini N, Nickerson LD, Beckmann CF et al (2012) Age-related adaptations of brain function during a memory task are also present at rest. Neuro Image 59:3821–3828. doi:10.1016/j.neuroimage.2011.11.063
Acknowledgements
We thank Mr. Bumwoo Park for assistance with imaging processing data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, and Family Affairs, Republic of Korea (HI12C1847).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Park, J.E., Jung, S.C., Ryu, K.H. et al. Differences in dynamic and static functional connectivity between young and elderly healthy adults. Neuroradiology 59, 781–789 (2017). https://doi.org/10.1007/s00234-017-1875-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1875-2