Abstract
Purpose
The risk of refractory epilepsy can be more dangerous than the adverse effect caused by medical treatment. In this study, we employed voxel-wise analysis (VWA) and tract-based spatial statistics (TBSS) methods to measure microstructural changes using diffusion tensor imaging (DTI) in patients of drug refractory epilepsy (DRE) who had been epileptic for more than 10 years.
Methods
To examine the specific microstructural abnormalities in DRE patients and its difference from medically controlled epilepsy (MCE), we acquired DTI data of 7 DRE patients, 37 MCE patients, and 31 healthy controls (HCs) using a 3 T MRI scanner. Comparisons between epileptic patients and HCs between MCE and DRE patients were performed based on calculated diffusion anisotropic indices data using VWA and TBSS.
Results
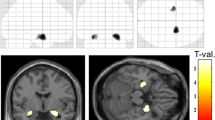
Compared to HCs, epileptic patients (including MCE and DRE) showed significant DTI changes in the common affected regions based on VWA, whereas TBSS found that widespread DTI changes in parts of microstructures of bilateral hemispheres were more obvious in the DRE patients than that in the MCE patients when compared with HCs. In contrast, significant reduction of fractional anisotropy values of thalamo-cortical fibers, including left superior temporal gyrus, insular cortex, pre-/post-central gyri, and thalamus, were further found in DRE patients compared with MCE.
Conclusion
The results of multiple diffusion anisotropic indices data provide complementary information to understand the dysfunction of thalamo-cortical pathway in DRE patients, which may be contributors to disorder of language and motor functions. Our current study may shed light on the pathophysiology of DRE.





Similar content being viewed by others
References
National Institute for Health and Clinical Excellence (2012) The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. National Clinical Guideline Centre, UK, pp 21–28
Stefan H, Theodore WH (2012) Epilepsy part I: basic principles and diagnosis, volume 107: handbook of clinical neurology. Elsevier, Amsterdam, p 471
Chen S, Wu X, Lui S, Wu Q, Yao Z, Li Q, Liang D, An D, Zhang X, Fang J, Huang X, Zhou D, Gong QY (2012) Resting-state fMRI study of treatment-naïve temporal lobe epilepsy patients with depressive symptoms. NeuroImage 60(1):299–304. doi:10.1016/j.neuroimage.2011.11.092
Wigg CM, Filgueiras A, Gomes Mda M (2014) The relationship between sleep quality, depression, and anxiety in patients with epilepsy and suicidal ideation. Arq Neuropsiquiatr 72(5):344–348
Eadie MJ (2012) Shortcomings in the current treatment of epilepsy. Expert Rev Neurother 12(12):1419–1427. doi:10.1586/ern.12.129
Hermann B, Meador KJ, Gaillard WD, Cramer JA (2010) Cognition across the lifespan: antiepileptic drugs, epilepsy, or both. Epilepsy Behav 17(1):1–5. doi:10.1016/j.yebeh.2009.10.019
Brunbech L, Sabers A (2002) Effect of antiepileptic drugs on cognitive function in individuals with epilepsy: a comparative review of newer versus older agents. Drugs 62(4):593–604
Hocker S, Wijdicks EF, Rabinstein AA (2013) Refractory status epilepticus: new insights in presentation, treatment, and outcome. Neurol Res 35(2):163–168. doi:10.1179/1743132812Y.0000000128
Viteva E (2013) Impact of stigma on the quality of life of patients with refractory epilepsy. Seizure 22(1):4–9. doi:10.1016/j.seizure.2012.10.010
McCagh J, Fisk JE, Baker GA (2009) Epilepsy, psychosocial and cognitive functioning. Epilepsy Res 86(1):1–14. doi:10.1016/j.eplepsyres.2009.04.007
Lee JJ, Song HS, Hwang YH, Lee HW, Suh CK, Park SP (2011) Psychiatric symptoms and quality of life in patients with drug-refractory epilepsy receiving adjunctive levetiracetam therapy. J Clin Neurol 7(3):128–136. doi:10.3988/jcn.2011.7.3.128
Zhong Y, Lu G, Zhang Z, Jiao Q, Li K, Liu Y (2011) Altered regional synchronization in epileptic patients with generalized tonic-clonic seizures. Epilepsy Res 97(1–2):83–91. doi:10.1016/j.eplepsyres.2011.07.007
Bernasconi N, Natsume J, Bernasconi A (2005) Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology 65(2):223–228. doi:10.1212/01.wnl.0000169066.46912.fa
Seidenberg M, Hermann B, Pulsipher D, Morton J, Parrish J, Geary E, Guidotti L (2008) Thalamic atrophy and cognition in unilateral temporal lobe epilepsy. J Int Neuropsychol Soc 14(3):384–393. doi:10.1017/S1355617708080399
Coan AC, Appenzeller S, Bonilha L, Li LM, Cendes F (2009) Seizure frequency and lateralization affect progression of atrophy in temporal lobe epilepsy. Neurology 73(11):834–842. doi:10.1212/WNL.0b013e3181b783dd
Cendes F, Andermann F, Dubeau F, Matthews PM, Arnold DL (1997) Normalization of neuronal metabolic dysfunction after surgery for temporal lobe epilepsy: evidence from 1H MR spectroscopic imaging. Neurology 49(6):1525–1533
Basser PJ, Jones DK (2002) Diffusion-tensor MRI: theory, experimental design and data analysis—a technical review. NMR Biomed 15(7–8):456–467. doi:10.1002/nbm.783
John A, Karl JF (2000) Voxel-based morphometry—the methods. NeuroImage 11:805–821
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31(4):1487–1505. doi:10.1016/j.neuroimage.2006.02.024
LeBihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13(4):534–546
Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66(1):259–267
Vaillancourt DE, Spraker MB, Prodoehl J, Abraham I, Corcos DM, Zhou XJ, Comella CL, Little DM (2009) High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology 72(16):1378–1384. doi:10.1212/01.wnl.0000340982.01727.6e
Feldman HM, Yeatman JD, Lee ES, Barde LH, Gaman-Bean S (2010) Diffusion tensor imaging: a review for pediatric researchers and clinicians. J Dev Behav Pediatr 31(4):346–356. doi:10.1097/DBP.0b013e3181dcaa8b
Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, do PM T, Zakszewski E, Field AS (2011) Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect 1(6):423–446. doi:10.1089/brain.2011.0071
do PM T (2016) DTI scalars (FA, MD, AD, RD)—how do they relate to brain structure? The Winnower 3:e146119.94778. doi:10.15200/winn.146119.94778
Fonov V, Evans AC, Botteron K, Almli CR, RC MK, Collins DL, Brain Development Cooperative Group (2011) Unbiased average age-appropriate atlases for pediatric studies. NeuroImage 54(1):313–327. doi:10.1016/j.neuroimage.2010.07.033
Liu W, Zhou Z, Liu X, Yan X, Yang G, Wang Z, Zhou Y, Bradly P, Xu D (2013) A slice-based optimization algorithm for diffusion tensors. (Chinese). Journal of Southern East University (Edition of Natural Science) 43(1):30–34. doi:10.3969/j.issn.1001-0505.2013.01.006
Bonelli SB, Powell RH, Yogarajah M, Samson RS, Symms MR, Thompson PJ, Koepp MJ, Duncan JS (2010) Imaging memory in temporal lobe epilepsy: predicting the effects of temporal lobe resection. Brain 133(Pt 4):1186–1199. doi:10.1093/brain/awq006
Köylü B, Walser G, Ischebeck A, Ortler M, Benke T (2008) Functional imaging of semantic memory predicts postoperative episodic memory functions in chronic temporal lobe epilepsy. Brain Res 1223:73–81. doi:10.1016/j.brainres.2008.05.075
Focke NK, Yogarajah M, Bonelli SB, Bartlett PA, Symms MR, Duncan JS (2008) Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. NeuroImage 40(2):728–737. doi:10.1016/j.neuroimage.2007.12.031
Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Barker GJ, Koepp MJ, Duncan JS (2007) Abnormalities of language networks in temporal lobe epilepsy. NeuroImage 36(1):209–221. doi:10.1016/j.neuroimage.2007.02.028
Byars AW, deGrauw TJ, Johnson CS, Perkins SM, Fastenau PS, Dunn DW, Austin JK (2014) Language and social functioning in children and adolescents with epilepsy. Epilepsy Behav 31:167–171. doi:10.1016/j.yebeh.2013.11.007
Price CJ (2010) The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci 1191:62–88. doi:10.1111/j.1749-6632.2010.05444.x
Kim J, Shin HK, Hwang KJ, Choi SJ, Joo EY, Hong SB, Hong SC, Seo DW (2014) Mirror focus in a patient with intractable occipital lobe epilepsy. J Epilepsy Res 4(1):34–37
Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R et al (2010) Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51(5):899–908. doi:10.1111/j.1528-1167.2010.02536.x
Avoli M, Kostopoulos G (1982) Participation of corticothalamic cells in penicillin-induced generalized spike and wave discharges. Brain Res 247(1):159–163
Bathelt J, Astle D, Barnes J, Raymond FL, Baker K (2016) Structural brain abnormalities in a single gene disorder associated with epilepsy, language impairment and intellectual disability. Neuroimage Clin 12:655–665. doi:10.1016/j.nicl.2016.07.016
Mesulam M (1985) Patterns in behavioral neuroanatomy: association areas, the limbic system, and hemispheric specialization. In: Mesulam M (ed) Principles of behavioral neurology. Philadelphia, pp 1–70
Gschwind M, Picard F (2016) Ecstatic epileptic seizures: a glimpse into the multiple roles of the insula. Front Behav Neurosci 10:1–22. doi:10.3389/fnbeh.2016.00021
Afzali M, Soltanian-Zadeh H, Elisevich KV (2011) Tract based spatial statistical analysis and voxel based morphometry of diffusion indices in temporal lobe epilepsy. Comput Biol Med 41(12):1082–1091. doi:10.1016/j.compbiomed.2011.05.006
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111(3):209–219
De Winter JCF (2013) Using the Student’s “t”-test with extremely small sample sizes. Practical Assessment, Research & Evaluation 18(10):1–12
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded in part by grants from the China National Natural Science Foundation (Nos. 81471734 and 31271188), a grant from the China National 13th Five-Year Program and a grant from the Shanghai Municipal Committee of Science and Technology (No. 13DJ1400300).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the Zhongshan Hospital affiliated with Fudan University Internal Review Board and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(PDF 141 kb)
Rights and permissions
About this article
Cite this article
Jiang, Y., Mao, L., Yan, X. et al. Investigation of altered microstructure in patients with drug refractory epilepsy using diffusion tensor imaging. Neuroradiology 59, 597–608 (2017). https://doi.org/10.1007/s00234-017-1835-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1835-x