Abstract
Introduction
We conducted a retrospective study to identify morphological subgroups of patients referred for AD or aMCI and to seek for differences across neuropsychological performances.
Methods
One hundred forty-five patients (mean age = 76.01, 88 women and 57 men) referred for AD, either at the stage of dementia or aMCI, were examined using structural MRI. Five observers reviewed blindly twice all examinations. We rated microangiopathy, hippocampal, parietal atrophies, including a gradient of fronto-parietal atrophy (GFPA). A multiple component analysis (MCA) followed by a hierarchical ascending classification was conducted to identify morphologically distinct subgroups. Among these, 76 patients completed all the neuropsychological tests. Univariate and multivariate analyses were further conducted on these data across morphological subgroups. The institutional review board approved the research protocol.
Results
Inter- and intra-raters’ agreements were excellent and very good for microangiopathy and hippocampal atrophy ratings. They were higher for GFPA than for the parietal atrophy scale.
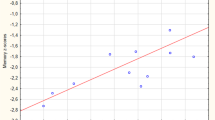
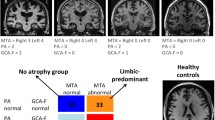
MCA without priors identified three groups: group 1 was characterized by no/discrete microangiopathy, severe hippocampal, and predominant parietal atrophy; group 2 had significant microangiopathy, severe hippocampal atrophy, and no predominant parietal atrophy; group 3 had a mild hippocampal atrophy and parietal atrophies. In group 1, working memory profile was less impaired than in group 2 (p = 0.01). Neuropsychological performances of group 3 were higher in most domains.
Conclusion
Combined characterization of microangiopathy, hippocampal, parietal, and GFPA allows identifying morphological subgroups among patients referred for AD and at risk. These groups have some neuropsychological differences, suggesting different pathophysiological mechanisms or co-existing conditions.


Similar content being viewed by others
References
Reitz C, Mayeux R (2014) Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88:640–651
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 7:263–269
Albert MS, DeKosky ST, Dickson D et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 7:270–279
Inzitari D, Pracucci G, Poggesi A et al (2009) Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ 339:b2477
Scheltens P, Leys D, Barkhof F et al (1992) Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 55:967–972
Dubois B, Feldman HH, Jacova C et al (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 6:734–746
Frisoni GB, Fox NC, Jack CR et al (2010) The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol 6:67–77
Du A-T, Schuff N, Kramer JH et al (2007) Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain J Neurol 130:1159–1166
Laakso MP, Partanen K, Riekkinen P et al (1996) Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: an MRI study. Neurology 46:678–681
Karas G, Scheltens P, Rombouts S et al (2007) Precuneus atrophy in early-onset Alzheimer’s disease: a morphometric structural MRI study. Neuroradiology 49:967–976
Bohnen NI, Djang DSW, Herholz K et al (2012) Effectiveness and safety of 18F-FDG PET in the evaluation of dementia: a review of the recent literature. J Nucl Med Off Publ Soc Nucl Med 53:59–71
Rowe CC, Villemagne VL (2011) Brain amyloid imaging. J Nucl Med Off Publ Soc Nucl Med 52:1733–1740
Johnson NA, Jahng G-H, Weiner MW et al (2005) Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology 234:851–859
Jacobs HIL, Van Boxtel MPJ, Jolles J et al (2012) Parietal cortex matters in Alzheimer’s disease: an overview of structural, functional and metabolic findings. Neurosci Biobehav Rev 36:297–309
Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain J Neurol 129:564–583
Mortamais M, Artero S, Ritchie K (2013) Cerebral white matter hyperintensities in the prediction of cognitive decline and incident dementia. Int Rev Psychiatry Abingdon Engl 25:686–698
Chutinet A, Rost NS (2014) White matter disease as a biomarker for long-term cerebrovascular disease and dementia. Curr Treat Options Cardiovasc Med 16:292
Tosto G, Zimmerman ME, Carmichael OT et al (2014) Predicting aggressive decline in mild cognitive impairment: the importance of white matter hyperintensities. JAMA 71:872–877
Koedam ELGE, Lehmann M, van der Flier WM et al (2011) Visual assessment of posterior atrophy development of a MRI rating scale. Eur Radiol 21:2618–2625
Signoret J-L (1991) BEM 144: batterie d’efficience mnésique 144. Fond. IPSEN Paris Elsevier Collect, Esprit Cerveau
Grober E, Buschke H, Crystal H et al (1988) Screening for dementia by memory testing. Neurology 38:900–903
Monaco M, Costa A, Caltagirone C, Carlesimo GA (2013) Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an Italian adult population. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 34:749–754
Bachy-Languedoc (1989) Batterie d’examen des troubles en dénomination. Brux. Ed.
Ashendorf L, Jefferson AL, O’Connor MK et al (2008) Trail making test errors in normal aging, mild cognitive impairment, and dementia. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol 23:129–137
Carlesimo GA, Caltagirone C, Gainotti G (1996) The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The group for the standardization of the mental deterioration battery. Eur Neurol 36:378–384
Herrera-Guzmán I, Peña-Casanova J, Lara JP et al (2004) Influence of age, sex, and education on the visual object and space perception battery (VOSP) in a healthy normal elderly population. Clin Neuropsychol 18:385–394
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Yoshita M, Fletcher E, Harvey D et al (2006) Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology 67:2192–2198
Jacobs HIL, Clerx L, Gronenschild EHBM et al (2014) White matter hyperintensities are positively associated with cortical thickness in Alzheimer’s disease. J Alzheimers Dis JAD 39:409–422
Capizzano AA, Ación L, Bekinschtein T et al (2004) White matter hyperintensities are significantly associated with cortical atrophy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 75:822–827
Ha S-Y, Youn YC, Kim S et al (2012) A voxel-based morphometric study of cortical gray matter volume changes in Alzheimer’s disease with white matter hyperintensities. J Clin Neurosci Off J Neurosurg Soc Australas 19:1506–1510
Seo SW, Lee J-M, Im K et al (2012) Cortical thinning related to periventricular and deep white matter hyperintensities. Neurobiol Aging 33:1156–1167
Au R, Massaro JM, Wolf PA et al (2006) Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham heart study. Arch Neurol 63:246–250
Oosterman JM, Van Harten B, Weinstein HC et al (2008) White matter hyperintensities and working memory: an explorative study. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 15:384–399
Schmidt R, Petrovic K, Ropele S et al (2007) Progression of leukoaraiosis and cognition. Stroke J Cereb Circ 38:2619–2625
Son SJ, Lee KS, Lee Y et al (2012) Association between white matter hyperintensity severity and cognitive impairment according to the presence of the apolipoprotein E (APOE) ε4 allele in the elderly: retrospective analysis of data from the CREDOS study. J Clin Psychiatry 73:1555–1562
Brickman AM, Siedlecki KL, Muraskin J et al (2011) White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol Aging 32:1588–1598
Sachdev P, Kalaria R, O’Brien J et al (2014) Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 28:206–218
Moulin CJA, James N, Freeman JE, Jones RW (2004) Deficient acquisition and consolidation: intertrial free recall performance in Alzheimer’s disease and mild cognitive impairment. J Clin Exp Neuropsychol 26:1–10
Kapeller P, Barber R, Vermeulen RJ et al (2003) Visual rating of age-related white matter changes on magnetic resonance imaging: scale comparison, interrater agreement, and correlations with quantitative measurements. Stroke J Cereb Circ 34:441–445
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
We declare that all human and animal studies have been approved by the CECIC Rhône-Alpes-Auvergne, Clermont-Ferrand (IRB 5891), and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chapuis, P., Sauvée, M., Medici, M. et al. Morphologic and neuropsychological patterns in patients suffering from Alzheimer’s disease. Neuroradiology 58, 459–466 (2016). https://doi.org/10.1007/s00234-016-1659-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-016-1659-0