Abstract
Introduction
Altered thalamocortical development is hypothesized to be a key substrate underlying neurodevelopmental disabilities in preterm infants. However, the pathogenesis of this abnormality is not well-understood. We combined magnetic resonance spectroscopy of the parietal white matter and morphometric analyses of the thalamus to investigate the association between white matter metabolism and thalamic volume and tested the hypothesis that thalamic volume would be associated with diminished N-acetyl-aspartate (NAA), a measure of neuronal/axonal maturation, independent of white matter injury.
Methods
Data from 106 preterm infants (mean gestational age at birth: 31.0 weeks ± 4.3; range 23–36 weeks) who underwent MR examinations under clinical indications were included in this study.
Results
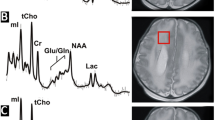
Linear regression analyses demonstrated a significant association between parietal white matter NAA concentration and thalamic volume. This effect was above and beyond the effect of white matter injury and age at MRI and remained significant even when preterm infants with punctate white matter lesions (pWMLs) were excluded from the analysis. Furthermore, choline, and among the preterm infants without pWMLs, lactate concentrations were also associated with thalamic volume. Of note, the associations between NAA and choline concentration and thalamic volume remained significant even when the sample was restricted to neonates who were term-equivalent age or older.
Conclusion
These observations provide convergent evidence of a neuroimaging phenotype characterized by widespread abnormal thalamocortical development and suggest that the pathogenesis may involve impaired axonal maturation.




Similar content being viewed by others
References
Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF (2010) The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 88:31–38
[2]. Aarnoudse-Moens CSH, Weisglas-Kuperus N, Goudoever JBv, Oosterlaan J (2009) Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children.
Marret S, Marchand-Martin L, Picaud JC, Hascoet JM, Arnaud C, Roze JC, Truffert P, Larroque B, Kaminski M, Ancel PY (2013) Brain injury in very preterm children and neurosensory and cognitive disabilities during childhood: the EPIPAGE cohort study. PLoS One 8:e62683
Volpe JJ (2009) Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8:110–124
Kostovic I, Judas M (2010) The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr 99:1119–1127
Marin-Padilla M (1970) Prenatal and early postnatal ontogenesis of the human motor cortex: a golgi study. I. The sequential development of the cortical layers. Brain Res 23:167–183
Boardman JP, Counsell SJ, Rueckert D, Kapellou O, Bhatia KK, Aljabar P, Hajnal J, Allsop JM, Rutherford MA, Edwards AD (2006) Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage 32:70–78
Srinivasan L, Dutta R, Counsell SJ, Allsop JM, Boardman JP, Rutherford MA, Edwards AD (2007) Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics 119:759–765
Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ (2005) Abnormal cerebral structure is present at term in premature infants. Pediatrics 115:286–294
Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, Jolesz F, Volpe JJ (1999) Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol 46:755–760
Boardman JP, Craven C, Valappil S, Counsell SJ, Dyet LE, Rueckert D, Aljabar P, Rutherford MA, Chew AT, Allsop JM, Cowan F, Edwards AD (2010) A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. Neuroimage 52:409–414
Ball G, Boardman JP, Aljabar P, Pandit A, Arichi T, Merchant N, Rueckert D, Edwards AD, Counsell SJ (2013) The influence of preterm birth on the developing thalamocortical connectome. Cortex 49:1711–1721
Ligam P, Haynes RL, Folkerth RD, Liu L, Yang M, Volpe JJ, Kinney HC (2009) Thalamic damage in periventricular leukomalacia: novel pathologic observations relevant to cognitive deficits in survivors of prematurity. Pediatr Res 65:524–529
Pierson CR, Folkerth RD, Billiards SS, Trachtenberg FL, Drinkwater ME, Volpe JJ, Kinney HC (2007) Gray matter injury associated with periventricular leukomalacia in the premature infant. Acta Neuropathol 114:619–631
Clark JB (1998) N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci 20:271–276
Patel TB, Clark JB (1979) Synthesis of N-acetyl-l-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. Biochem J 184:539–546
Patel TB, Clark JB (1980) Lipogenesis in the brain of suckling rats. Studies on the mechansim of mitochondrial-cytosolic carbon transfer. Biochem J 188:163–168
Bhakoo KK, Williams IT, Williams SR, Gadian DG, Noble MD (1996) Proton nuclear magnetic resonance spectroscopy of primary cells derived from nervous tissue. J Neurochem 66:1254–1263
Burri R, Steffen C, Herschkowitz N (1991) N-acetyl-L-aspartate is a major source of acetyl groups for lipid synthesis during rat brain development. Dev Neurosci 13:403–411
Bluml S, Wisnowski JL, Nelson MD Jr, Paquette L, Gilles FH, Kinney HC, Panigrahy A (2013) Metabolic maturation of the human brain from birth through adolescence: insights from in vivo magnetic resonance spectroscopy. Cereb Cortex 23:2944–2955
Xu D, Bonifacio S, Charlton NN, Vaughan CP, Lu Y, Ferriero DM, Vigneron DB, Barkovich AJ (2011) MR spectroscopy of normative premature newborns. J Magn Reson Imaging 33:306–311
Limperopoulos C, Tworetzky W, McElhinney DB, Newburger JW, Brown DW, Robertson RL Jr, Guizard N, McGrath E, Geva J, Annese D, Dunbar-Masterson C, Trainor B, Laussen PC, du Plessis AJ (2010) Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 121:26–33
Wisnowski JL, Bluml S, Paquette L, Zelinski E, Nelson MD Jr, Painter MJ, Damasio H, Gilles F, Panigrahy A (2013) Altered glutamatergic metabolism associated with punctate white matter lesions in preterm infants. PLoS One 8:e56880
Ross BD, Ernst T, Kreis R, Haseler LJ, Bayer S, Danielsen E, Bluml S, Shonk T, Mandigo JC, Caton W, Clark C, Jensen SW, Lehman NL, Arcinue E, Pudenz R, Shelden CH (1998) 1H MRS in acute traumatic brain injury. J Magn Reson Imaging 8:829–840
Gideon P, Henriksen O, Sperling BK, Christiansen P, Olsen TS, Jorgensen HS, Arlien-Soborg P (1993) Magnetic resonance spectroscopy of acute cerebral infarctions. Ugeskr Laeger 155:3194–3199
Barkovich AJ, Baranski K, Vigneron D, Partridge JC, Hallam DK, Hajnal BL, Ferriero DM (1999) Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol 20:1399–1405
Cady EB, Penrice J, Amess PN, Lorek A, Wylezinska M, Aldridge RF, Franconi F, Wyatt JS, Reynolds EO (1996) Lactate, N-acetylaspartate, choline and creatine concentrations, and spin-spin relaxation in thalamic and occipito-parietal regions of developing human brain. Magn Reson Med 36:878–886
Johnston MV (2005) Excitotoxicity in perinatal brain injury. Brain Pathol 15:234–240
Vannucci SJ, Hagberg H (2004) Hypoxia-ischemia in the immature brain. J Exp Biol 207:3149–3154
Bluml S, Seymour KJ, Ross BD (1999) Developmental changes in choline- and ethanolamine-containing compounds measured with proton-decoupled (31)P MRS in in vivo human brain. Magn Reson Med 42:643–654
Miller BL, Chang L, Booth R, Ernst T, Cornford M, Nikas D, McBride D, Jenden DJ (1996) In vivo 1H MRS choline: correlation with in vitro chemistry/histology. Life Sci 58:1929–1935
Chang L, Munsaka SM, Kraft-Terry S, Ernst T (2013) Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol 8:576–593
Wisnowski JL, Schmithorst VJ, Rosser T, Paquette L, Nelson MD, Haynes RL, Painter MJ, Bluml S, Panigrahy A (2014) Magnetic resonance spectroscopy markers of axons and astrogliosis in relation to specific features of white matter injury in preterm infants. Neuroradiology 56:771–779
Kinney HC (2009) The encephalopathy of prematurity: one pediatric neuropathologist's perspective. Semin Pediatr Neurol 16:179–190
Haynes RL, Borenstein NS, Desilva TM, Folkerth RD, Liu LG, Volpe JJ, Kinney HC (2005) Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol 484:156–167
Volpe JJ (1998) Brain injury in the premature infant: overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol 5:135–151
Maalouf E, Duggan P, Rutherford M, Counsell S, Fletcher A, Battin M, Cowan F, Edwards A (1999) Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. J Pediatr 135:351–357
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 31:1116–1128
[39]. Morel A (2013) Stereotactic atlas of the human thalamus and basal ganglia.
Bluml S, Wisnowski JL, Nelson MD Jr, Paquette L, Panigrahy A (2014) Metabolic maturation of white matter is altered in preterm infants. PLoS One 9:e85829
Cohen J (1988) Statistical power analysis for the behavioral sciences. L. Erlbaum Associates, Hillsdale
Ball G, Boardman JP, Rueckert D, Aljabar P, Arichi T, Merchant N, Gousias IS, Edwards AD, Counsell SJ (2012) The effect of preterm birth on thalamic and cortical development. Cereb Cortex 22:1016–1024
Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA (2011) The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int J Dev Neurosci 29:423–440
Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR, Back SA (2012) Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol 71:93–109
Dezortova M, Hajek M (2008) (1)H MR spectroscopy in pediatrics. Eur J Radiol 67:240–249
Kok RD, van den Berg PP, van den Bergh AJ, Nijland R, Heerschap A (2002) Maturation of the human fetal brain as observed by 1H MR spectroscopy. Magn Reson Med 48:611–616
Girard N, Fogliarini C, Viola A, Confort-Gouny S, Fur YL, Viout P, Chapon F, Levrier O, Cozzone P (2006) MRS of normal and impaired fetal brain development. Eur J Radiol 57:217–225
Bitsch A, Bruhn H, Vougioukas V, Stringaris A, Lassmann H, Frahm J, Bruck W (1999) Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. AJNR Am J Neuroradiol 20:1619–1627
Brenner RE, Munro PM, Williams SC, Bell JD, Barker GJ, Hawkins CP, Landon DN, McDonald WI (1993) The proton NMR spectrum in acute EAE: the significance of the change in the Cho:Cr ratio. Magn Reson Med 29:737–745
Degaonkar MN, Khubchandhani M, Dhawan JK, Jayasundar R, Jagannathan NR (2002) Sequential proton MRS study of brain metabolite changes monitored during a complete pathological cycle of demyelination and remyelination in a lysophosphatidyl choline (LPC)-induced experimental demyelinating lesion model. NMR Biomed 15:293–300
Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC (2001) Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 21:1302–1312
Billiards SS, Haynes RL, Folkerth RD, Borenstein NS, Trachtenberg FL, Rowitch DH, Ligon KL, Volpe JJ, Kinney HC (2008) Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol 18:153–163
Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A, Craig A, Struve J, Sherman LS, Back SA (2008) Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol 63:520–530
Alix JJ, Zammit C, Riddle A, Meshul CK, Back SA, Valentino M, Fern R (2012) Central axons preparing to myelinate are highly sensitivity to ischemic injury. Ann Neurol 72:936–951
Sanchez I, Hassinger L, Paskevich PA, Shine HD, Nixon RA (1996) Oligodendroglia regulate the regional expansion of axon caliber and local. J Neurosci 16:5095–5105
Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, Li L, Ji X, Trommer B, Beardsley DJ, Luo NL, Back SA, Tan S (2005) Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci 25:5988–5997
Bartha AI, Foster-Barber A, Miller SP, Vigneron DB, Glidden DV, Barkovich AJ, Ferriero DM (2004) Neonatal encephalopathy: association of cytokines with MR spectroscopy and outcome. Pediatr Res 56:960–966
Pavlakis SG, Lu D, Frank Y, Wiznia A, Eidelberg D, Barnett T, Hyman RA (1998) Brain lactate and N-acetylaspartate in pediatric AIDS encephalopathy. AJNR Am J Neuroradiol 19:383–385
Kim S, Steelman AJ, Zhang Y, Kinney HC, Li J (2012) Aberrant upregulation of astroglial ceramide potentiates oligodendrocyte injury. Brain Pathol 22:41–57
Billiards SS, Haynes RL, Folkerth RD, Trachtenberg FL, Liu LG, Volpe JJ, Kinney HC (2006) Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol 497:199–208
Dyet L, Kennea N, Counsell S, Maalouf E, Ajayi-Obe M, Duggan P, Harrison M, Allsop J, Hajnal J, Herlihy A, Edwards B, Laroche S, Cowan F, Rutherford M, Edwards A (2006) Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics 118:536–548
Jeon TY, Kim JH, Yoo SY, Eo H, Kwon JY, Lee J, Lee M, Chang YS, Park WS (2012) Neurodevelopmental outcomes in preterm infants: comparison of infants with and without diffuse excessive high signal intensity on MR images at near-term-equivalent age. Radiology 263:518–526
Kidokoro H, Anderson PJ, Doyle LW, Neil JJ, Inder TE (2011) High signal intensity on T2-weighted MR imaging at term-equivalent age in preterm infants does not predict 2-year neurodevelopmental outcomes. AJNR Am J Neuroradiol 32:2005–2010
Raybaud C, Ahmad T, Rastegar N, Shroff M, Al Nassar M (2013) The premature brain: developmental and lesional anatomy. Neuroradiology 55(Suppl 2):23–40
Chau V, Synnes A, Grunau RE, Poskitt KJ, Brant R, Miller SP (2013) Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology 81:2082–2089
Bapat R, Narayana P, Zhou Y, Parikh N (2014) Magnetic resonance spectroscopy at term equivalent age in extremely preterm infants: association with cognitive and language development. Pediatr Neurol 51:53–59
Kendall GS, Melbourne A, Johnson S, Price D, Bainbridge A, Gunny R, Huertas-Ceballos A, Cady EB, Ourselin S, Marlow N, Robertson NJ (2014) White matter NAA/Cho and Cho/Cr ratios at MR spectroscopy are predictive of motor outcome in preterm infants. Radiology 271:230–238
Augustine EM, Spielman DM, Barnes PD, Sutcliffe TL, Dermon JD, Mirmiran M, Clayton DB, Ariagno RL (2008) Can magnetic resonance spectroscopy predict neurodevelopmental outcome in very low birth weight preterm infants? J Perinatol 28:611–618
Acknowledgments
The authors would like to thank Julia Castro for organizing the data, the NICU and Radiology staff at Children’s Hospital Los Angeles, and the families for their participation in our research studies.
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the Children’s Hospital Los Angeles Committee on Clinical Investigations and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in the study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wisnowski, J.L., Ceschin, R.C., Choi, S.Y. et al. Reduced thalamic volume in preterm infants is associated with abnormal white matter metabolism independent of injury. Neuroradiology 57, 515–525 (2015). https://doi.org/10.1007/s00234-015-1495-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1495-7