Abstract
Introduction
The aim of this study was to prospectively investigate whether the structure of cerebral small-resistance arteries is related to cerebral perfusion parameters as measured with dynamic susceptibility-weighted contrast magnetic resonance imaging (DSC-MRI) in a selected cohort of hypertensive and normotensive patients.
Methods
Ten hypertensive and 10 normotensive patients were included in the study. All patients underwent neurosurgical intervention for an intracranial tumor and were investigated with DSC-MRI at 1.5 T. Cerebral small-resistance arteries were dissected from a small portion of morphologically normal cerebral tissue and mounted on an isometric myograph for the measurement of the media-to-lumen (M/L) ratio. A quantitative assessment of cerebral blood flow (CBF) and volume (CBV) was performed with a region-of-interest approach. Correlation coefficients were calculated for normally distributed variables. The institutional review board approved the study, and informed consent was obtained from all patients.
Results
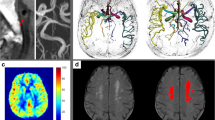
Compared with normotensive subjects, hypertensive patients had significantly lower regional CBF (mL/100 g/min) in the cortical grey matter (55.63 ± 1.90 vs 58.37 ± 2.19, p < 0.05), basal ganglia (53.34 ± 4.39 vs 58.22. ± 4.33, p < 0.05), thalami (50.65 ± 3.23 vs 57.56 ± 4.45, p < 0.01), subcortical white matter (19.32 ± 2.54 vs 22.24 ± 1.9, p < 0.05), greater M/L ratio (0.099 ± 0.013 vs 0.085 ± 0.012, p < 0.05), and lower microvessel density (1.66 ± 0.67 vs 2.52 ± 1.28, p < 0.05). A statistically significant negative correlation was observed between M/L ratio of cerebral arteries and CBF in the cortical grey matter (r = −0.516, p < 0.05), basal ganglia (r = −0.521, p < 0.05), thalami (r = −0.527 p < 0.05), and subcortical white matter (r = −0.612, p < 0.01).
Conclusion
Our results indicate that microvascular structure might play a role in controlling CBF, with possible clinical consequences.





Similar content being viewed by others
References
Folkow B (1982) Physiological aspects of primary hypertension. Physiol Rev 62:347–504
Mulvany MJ, Aalkjaer C (1990) Structure and function of small arteries. Physiol Rev 70:921–961
Rizzoni D, Porteri E, Castellano M, Bettoni G, Muiesan ML, Muiesan P, Giulini SM, Agabiti-Rosei E (1996) Vascular hypertrophy and remodeling in secondary hypertension. Hypertension 28:785–790
Levy BI, Ambrosio G, Pries AR, Struijker-Boudier HA (2001) Microcirculation in hypertension: a new target for treatment? Circulation 104:735–740
Mulvany MJ (2002) Small artery remodeling and significance in the development of hypertension. News Physiol Sci 17:105–109
Park JB, Schiffrin EL (2001) Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J Hypertens 19:921–930
De CC, Porteri E, Rizzoni D, Rizzardi N, Paiardi S, Boari GE, Miclini M, Zani F, Muiesan ML, Donato F, Salvetti M, Castellano M, Tiberio GA, Giulini SM, Agabiti RE (2007) Structural alterations of subcutaneous small-resistance arteries may predict major cardiovascular events in patients with hypertension. Am J Hypertens 20:846–852
Mathiassen ON, Buus NH, Olsen HW, Larsen ML, Mulvany MJ, Christensen KL (2006) Forearm plethysmography in the assessment of vascular tone and resistance vasculature design: new methodological insights. Acta Physiol (Oxford) 188:91–101
Rizzoni D, Porteri E, Boari GE, De CC, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E (2003) Prognostic significance of small-artery structure in hypertension. Circulation 108:2230–2235
Agabiti-Rosei E, Rizzoni D (2007) The effects of hypertension on the structure of human resistance arteries. In: Lip GYH, Hall JE (eds) Comprehensive hypertension. Mosby Elsevier, pp 579–590
Rizzoni D, De CC, Porteri E, Paiardi S, Boari GE, Mortini P, Cornali C, Cenzato M, Rodella LF, Borsani E, Rizzardi N, Platto C, Rezzani R, Rosei EA (2009) Altered structure of small cerebral arteries in patients with essential hypertension. J Hypertens 27:838–845
Novak V, Chowdhary A, Farrar B, Nagaraja H, Braun J, Kanard R, Novak P, Slivka A (2003) Altered cerebral vasoregulation in hypertension and stroke. Neurology 60:1657–1663
Shaw TG, Mortel KF, Meyer JS, Rogers RL, Hardenberg J, Cutaia MM (1984) Cerebral blood flow changes in benign aging and cerebrovascular disease. Neurology 34:855–862
Nobili F, Rodriguez G, Marenco S, De CF, Gambaro M, Castello C, Pontremoli R, Rosadini G (1993) Regional cerebral blood flow in chronic hypertension. A correlative study. Stroke 24:1148–1153
Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM (2008) Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke 39:349–354
Waldstein SR, Lefkowitz DM, Siegel EL, Rosenberger WF, Spencer RJ, Tankard CF, Manukyan Z, Gerber EJ, Katzel L (2010) Reduced cerebral blood flow in older men with higher levels of blood pressure. J Hypertens 28:993–998
Beason-Held LL, Moghekar A, Zonderman AB, Kraut MA, Resnick SM (2007) Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke 38:1766–1773
Appelman AP, van der Graaf Y, Vincken KL, Tiehuis AM, Witkamp TD, Mali WP, Geerlings MI (2008) Total cerebral blood flow, white matter lesions and brain atrophy: the SMART-MR study. J Cereb Blood Flow Metab 28:633–639
Geerlings MI, Appelman AP, Vincken KL, Algra A, Witkamp TD, Mali WP, van der Graaf Y (2010) Brain volumes and cerebrovascular lesions on MRI in patients with atherosclerotic disease. The SMART-MR study. Atherosclerosis 210:130–136
Muller M, van der Graaf Y, Visseren FL, Mali WP, Geerlings MI (2012) Hypertension and longitudinal changes in cerebral blood flow: the SMART-MR study. Ann Neurol 71:825–833
Mancia G, De BG, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A (2007) 2007 ESH-ESC Practice Guidelines for the management of arterial hypertension: ESH-ESC Task Force on the management of arterial hypertension. J Hypertens 25:1751–1762
Rizzoni D, Porteri E, Guelfi D, Muiesan ML, Valentini U, Cimino A, Girelli A, Rodella L, Bianchi R, Sleiman I, Rosei EA (2001) Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin-dependent diabetes mellitus. Circulation 103:1238–1244
Munzenmaier DH, Greene AS (2006) Chronic angiotensin II AT1 receptor blockade increases cerebral cortical microvessel density. Am J Physiol Heart Circ Physiol 290:H512–H516
Ostergaard L, Sorensen AG, Kwong KK, Weisskoff RM, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: experimental comparison and preliminary results. Magn Reson Med 36:726–736
Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: mathematical approach and statistical analysis. Magn Reson Med 36:715–725
Wu O, Ostergaard L, Weisskoff RM, Benner T, Rosen BR, Sorensen AG (2003) Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med 50:164–174
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27:859–867
Heagerty AM (2007) Predicting hypertension complications from small artery structure. J Hypertens 25:939–940
Wardlaw JM (2005) What causes lacunar stroke? J Neurol Neurosurg Psychiatry 76:617–619
Jackson CA, Hutchison A, Dennis MS, Wardlaw JM, Lindgren A, Norrving B, Anderson CS, Hankey GJ, Jamrozik K, Appelros P, Sudlow CL (2010) Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke 41:624–629
de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, Van GJ, Breteler MM (2002) Hypertension and cerebral white matter lesions in a prospective cohort study. Brain 125:765–772
Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D'Agostino RB, DeCarli C (2004) Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke 35:1857–1861
Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O'Brien JT, Ford GA (2007) Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure. J Neurol 254:713–721
O'Sullivan M, Lythgoe DJ, Pereira AC, Summers PE, Jarosz JM, Williams SC, Markus HS (2002) Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology 59:321–326
Kimura Y, Kitagawa K, Oku N, Kajimoto K, Kato H, Tanaka M, Sakaguchi M, Hougaku H, Sakoda S, Hatazawa J (2010) Blood pressure lowering with valsartan is associated with maintenance of cerebral blood flow and cerebral perfusion reserve in hypertensive patients with cerebral small vessel disease. J Stroke Cerebrovasc Dis 19:85–91
Nagata R, Kawabe K, Ikeda K (2010) Olmesartan, an angiotensin II receptor blocker, restores cerebral hypoperfusion in elderly patients with hypertension. J Stroke Cerebrovasc Dis 19:236–240
Lipsitz LA, Gagnon M, Vyas M, Iloputaife I, Kiely DK, Sorond F, Serrador J, Cheng DM, Babikian V, Cupples LA (2005) Antihypertensive therapy increases cerebral blood flow and carotid distensibility in hypertensive elderly subjects. Hypertension 45:216–221
Zhang R, Witkowski S, Fu Q, Claassen JA, Levine BD (2007) Cerebral hemodynamics after short- and long-term reduction in blood pressure in mild and moderate hypertension. Hypertension 49:1149–1155
Tryambake D, He J, Firbank MJ, O'Brien JT, Blamire AM, Ford GA (2013) Intensive blood pressure lowering increases cerebral blood flow in older subjects with hypertension. Hypertension 61:1309–1315
Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R (1999) Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab 19:701–735
Rempp KA, Brix G, Wenz F, Becker CR, Guckel F, Lorenz WJ (1994) Quantification of regional cerebral blood flow and volume with dynamic susceptibility contrast-enhanced MR imaging. Radiology 193:637–641
Vonken EJ, van Osch MJ, Bakker CJ, Viergever MA (1999) Measurement of cerebral perfusion with dual-echo multi-slice quantitative dynamic susceptibility contrast MRI. J Magn Reson Imaging 10:109–117
Ziegelitz D, Starck G, Mikkelsen IK, Tullberg M, Edsbagge M, Wikkelso C, Forssell-Aronson E, Holtas S, Knutsson L (2009) Absolute quantification of cerebral blood flow in neurologically normal volunteers: dynamic-susceptibility contrast MRI-perfusion compared with computed tomography (CT)-perfusion. Magn Reson Med 62:56–65
Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S (1990) Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113(Pt 1):27–47
Shin W, Horowitz S, Ragin A, Chen Y, Walker M, Carroll TJ (2007) Quantitative cerebral perfusion using dynamic susceptibility contrast MRI: evaluation of reproducibility and age- and gender-dependence with fully automatic image postprocessing algorithm. Magn Reson Med 58:1232–1241
Knutsson L, Stahlberg F, Wirestam R (2010) Absolute quantification of perfusion using dynamic susceptibility contrast MRI: pitfalls and possibilities. MAGMA 23:1–21
Pantoni L (2010) Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 9:689–701
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the University of Brescia Medical School Ethics Committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Acknowledgments
This study was partly financed by the European Community’s Sixth Framework Program “InGenious HyperCare”.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
De Ciuceis, C., Cornali, C., Porteri, E. et al. Cerebral small-resistance artery structure and cerebral blood flow in normotensive subjects and hypertensive patients. Neuroradiology 56, 1103–1111 (2014). https://doi.org/10.1007/s00234-014-1423-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1423-2