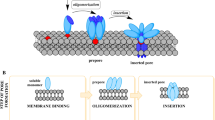
Abstract
Pore-forming protein toxins (PFTs) represent a diverse class of membrane-damaging proteins that are produced by a wide variety of organisms. PFT-mediated membrane perforation is largely governed by the chemical composition and the physical properties of the plasma membranes. The interaction between the PFTs with the target membranes is critical for the initiation of the pore-formation process, and can lead to discrete membrane reorganization events that further aids in the process of pore-formation. Punching holes on the plasma membranes by the PFTs interferes with the cellular homeostasis by disrupting the ion-balance inside the cells that in turn can turn on multiple signalling cascades required to restore membrane integrity and cellular homeostasis. In this review, we discuss the physicochemical attributes of the plasma membranes associated with the pore-formation processes by the PFTs, and the subsequent membrane remodelling events that may start off the membrane-repair mechanisms.
Graphic abstract





Similar content being viewed by others
References
Adams CJ, Kopp MC, Larburu N, Nowak PR, Ali MMU (2019) Structure and molecular mechanism of ER stress signaling by the unfolded protein response signal activator IRE1. Front Mol Biosci 6:11
Alhamdi Y, Neill DR, Abrams ST, Malak HA, Yahya R, Barrett-Jolley R, Wang G, Kadioglu A, Toh CH (2015) Circulating pneumolysin is a potent inducer of cardiac injury during pneumococcal infection. PLoS Pathog 11:e1004836
Alouf JE, Geoffroy C, Pattus F, Verger R (1984) Surface properties of bacterial sulfhydryl-activated cytolytic toxins. Interaction with monomolecular films of phosphatidylcholine and various sterols. Eur J Biochem 141:205–210
Alvarez C, Mancheno JM, Martinez D, Tejuca M, Pazos F, Lanio ME (2009) Sticholysins, two pore-forming toxins produced by the Caribbean sea anemone Stichodactyla helianthus: their interaction with membranes. Toxicon 54:1135–1147
Alvarez C, Ros U, Valle A, Pedrera L, Soto C, Hervis YP, Cabezas S, Valiente PA, Pazos F, Lanio ME (2017) Biophysical and biochemical strategies to understand membrane binding and pore formation by sticholysins, pore-forming proteins from a sea anemone. Biophys Rev 9:529–544
Atanassoff AP, Wolfmeier H, Schoenauer R, Hostettler A, Ring A, Draeger A, Babiychuk EB (2014) Microvesicle shedding and lysosomal repair fulfill divergent cellular needs during the repair of streptolysin O-induced plasmalemmal damage. PLoS One 9:e89743
Aufderhorst-Roberts A, Chandra U, Connell SD (2017) Three-phase coexistence in lipid membranes. Biophys J 112:313–324
Babiychuk EB, Monastyrskaya K, Potez S, Draeger A (2009) Intracellular Ca(2+) operates a switch between repair and lysis of streptolysin O-perforated cells. Cell Death Differ 16:1126–1134
Babiychuk EB, Monastyrskaya K, Potez S, Draeger A (2011) Blebbing confers resistance against cell lysis. Cell Death Differ 18:80–89
Bayley H, Jayasinghe L, Wallace M (2005) Prepore for a breakthrough. Nat Struct Mol Biol 12:385–386
Bernardes N, Fialho AM (2018) Perturbing the dynamics and organization of cell membrane components: a new paradigm for cancer-targeted therapies. Int J Mol Sci 19:9871
Bischof LJ, Kao CY, Los FC, Gonzalez MR, Shen Z, Briggs SP, van der Goot FG, Aroian RV (2008) Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog 4:e1000176
Bonev BB, Lam YH, Anderluh G, Watts A, Norton RS, Separovic F (2003) Effects of the eukaryotic pore-forming cytolysin equinatoxin II on lipid membranes and the role of sphingomyelin. Biophys J 84:2382–2392
Boyd CM, Parsons ES, Smith RA, Seddon JM, Ces O, Bubeck D (2016) Disentangling the roles of cholesterol and CD59 in intermedilysin pore formation. Sci Rep 6:38446
Brown DA, London E (2000) Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 275:17221–17224
Broz P, Pelegrin P, Shao F (2020) The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol 20:143–157
Cabezas S, Ho S, Ros U, Lanio ME, Alvarez C, van der Goot FG (2017) Damage of eukaryotic cells by the pore-forming toxin sticholysin II: consequences of the potassium efflux. Biochim Biophys Acta Biomembr 1859:982–992
Castro-Gomes T, Corrotte M, Tam C, Andrews NW (2016) Plasma membrane repair is regulated extracellularly by proteases released from lysosomes. PLoS One 11:e1052583
Dal Peraro M, van der Goot FG (2016) Pore-forming toxins: ancient, but never really out of fashion. Nat Rev Microbiol 14:77–92
Daleke DL (2003) Regulation of transbilayer plasma membrane phospholipid asymmetry. J Lipid Res 44:233–242
Diep DB, Nelson KL, Raja SM, Pleshak EN, Buckley JT (1998) Glycosylphosphatidylinositol anchors of membrane glycoproteins are binding determinants for the channel-forming toxin aerolysin. J Biol Chem 273:2355–2360
Donato M, Soto C, Lanio ME, Itri R, Alvarez C (2021) The pore-forming activity of sticholysin I is enhanced by the presence of a phospholipid hydroperoxide in membrane. Toxicon 204:44–55
Drucker P, Iacovache I, Bachler S, Zuber B, Babiychuk EB, Dittrich PS, Draeger A (2019) Membrane deformation and layer-by-layer peeling of giant vesicles induced by the pore-forming toxin pneumolysin. Biomater Sci 7:3693–3705
Etxaniz A, Gonzalez-Bullon D, Martin C, Ostolaza H (2018) Membrane repair mechanisms against permeabilization by pore-forming toxins. Toxins (Basel) 10:234
Evans JC, Johnstone BA, Lawrence SL, Morton CJ, Christie MP, Parker MW, Tweten RK (2020) A key motif in the cholesterol-dependent cytolysins reveals a large family of related proteins. mBio. https://doi.org/10.1128/mBio.02351-20
Farrand AJ, LaChapelle S, Hotze EM, Johnson AE, Tweten RK (2010) Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc Natl Acad Sci U S A 107:4341–4346
Flanagan JJ, Tweten RK, Johnson AE, Heuck AP (2009) Cholesterol exposure at the membrane surface is necessary and sufficient to trigger perfringolysin O binding. Biochemistry 48:3977–3987
Fuertes G, Gimenez D, Esteban-Martin S, Garcia-Saez A, Sanchez O, Salgado J (2010) Role of membrane lipids for the activity of pore forming peptides and proteins. Adv Exp Med Biol 677:31–55
Garcia-Ortega L, Alegre-Cebollada J, Garcia-Linares S, Bruix M, Martinez-Del-Pozo A, Gavilanes JG (2011) The behavior of sea anemone actinoporins at the water-membrane interface. Biochim Biophys Acta 1808:2275–2288
Garcia-Saez AJ, Buschhorn SB, Keller H, Anderluh G, Simons K, Schwille P (2011) Oligomerization and pore formation by equinatoxin II inhibit endocytosis and lead to plasma membrane reorganization. J Biol Chem 286:37768–37777
Garcia-Saez AJ, Chiantia S, Salgado J, Schwille P (2007a) Pore formation by a Bax-derived peptide: effect on the line tension of the membrane probed by AFM. Biophys J 93:103–112
Garcia-Saez AJ, Chiantia S, Schwille P (2007b) Effect of line tension on the lateral organization of lipid membranes. J Biol Chem 282:33537–33544
Gekara NO, Jacobs T, Chakraborty T, Weiss S (2005) The cholesterol-dependent cytolysin listeriolysin O aggregates rafts via oligomerization. Cell Microbiol 7:1345–1356
Giddings KS, Johnson AE, Tweten RK (2003) Redefining cholesterol’s role in the mechanism of the cholesterol-dependent cytolysins. Proc Natl Acad Sci U S A 100:11315–11320
Gilbert RJ (2016) Protein-lipid interactions and non-lamellar lipidic structures in membrane pore formation and membrane fusion. Biochim Biophys Acta 1858:487–499
Gonzalez MR, Bischofberger M, Freche B, Ho S, Parton RG, van der Goot FG (2011) Pore-forming toxins induce multiple cellular responses promoting survival. Cell Microbiol 13:1026–1043
Gonzalez MR, Bischofberger M, Pernot L, van der Goot FG, Freche B (2008) Bacterial pore-forming toxins: the (w)hole story? Cell Mol Life Sci 65:493–507
Gordon VM, Klimpel KR, Arora N, Henderson MA, Leppla SH (1995) Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect Immun 63:82–87
Haney EF, Nathoo S, Vogel HJ, Prenner EJ (2010) Induction of non-lamellar lipid phases by antimicrobial peptides: a potential link to mode of action. Chem Phys Lipids 163:82–93
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13:89–102
Hong Y, Ohishi K, Inoue N, Kang JY, Shime H, Horiguchi Y, van der Goot FG, Sugimoto N, Kinoshita T (2002) Requirement of N-glycan on GPI-anchored proteins for efficient binding of aerolysin but not Clostridium septicum alpha-toxin. EMBO J 21:5047–5056
Husmann M, Beckmann E, Boller K, Kloft N, Tenzer S, Bobkiewicz W, Neukirch C, Bayley H, Bhakdi S (2009) Elimination of a bacterial pore-forming toxin by sequential endocytosis and exocytosis. FEBS Lett 583:337–344
Iacovache I, Bischofberger M, van der Goot FG (2010) Structure and assembly of pore-forming proteins. Curr Opin Struct Biol 20:241–246
Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW (2008) Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J Cell Biol 180:905–914
Ilangumaran Ponmalar I, Sarangi NK, Basu JK, Ayappa KG (2021) Pore forming protein induced biomembrane reorganization and dynamics: a focused review. Front Mol Biosci 8:737561
Ivie SE, McClain MS (2012) Identification of amino acids important for binding of Clostridium perfringens epsilon toxin to host cells and to HAVCR1. Biochemistry 51:7588–7595
Jimenez AJ, Perez F (2017) Plasma membrane repair: the adaptable cell life-insurance. Curr Opin Cell Biol 47:99–107
Jimenez AJ, Maiuri P, Lafaurie-Janvore J, Divoux S, Piel M, Perez F (2014) ESCRT machinery is required for plasma membrane repair. Science 343:1247136
Johnson CL, Ridley H, Marchetti R, Silipo A, Griffin DC, Crawford L, Bonev B, Molinaro A, Lakey JH (2014) The antibacterial toxin colicin N binds to the inner core of lipopolysaccharide and close to its translocator protein. Mol Microbiol 92:440–452
Karatekin E, Sandre O, Guitouni H, Borghi N, Puech PH, Brochard-Wyart F (2003) Cascades of transient pores in giant vesicles: line tension and transport. Biophys J 84:1734–1749
Kathuria R, Chattopadhyay K (2018) Vibrio cholerae cytolysin: multiple facets of the membrane interaction mechanism of a beta-barrel pore-forming toxin. IUBMB Life 70:260–266
Kathuria R, Mondal AK, Sharma R, Bhattacharyya S, Chattopadhyay K (2018) Revisiting the role of cholesterol in regulating the pore-formation mechanism of Vibrio cholerae cytolysin, a membrane-damaging beta-barrel pore-forming toxin. Biochem J 475:3039–3055
Kaus K, Lary JW, Cole JL, Olson R (2014) Glycan specificity of the Vibrio vulnificus hemolysin lectin outlines evolutionary history of membrane targeting by a toxin family. J Mol Biol 426:2800–2812
Keyel PA, Loultcheva L, Roth R, Salter RD, Watkins SC, Yokoyama WM, Heuser JE (2011) Streptolysin O clearance through sequestration into blebs that bud passively from the plasma membrane. J Cell Sci 124:2414–2423
Kulma M, Anderluh G (2021) Beyond pore formation: reorganization of the plasma membrane induced by pore-forming proteins. Cell Mol Life Sci 78:6229–6249
Kulma M, Herec M, Grudzinski W, Anderluh G, Gruszecki WI, Kwiatkowska K, Sobota A (2010) Sphingomyelin-rich domains are sites of lysenin oligomerization: implications for raft studies. Biochim Biophys Acta 1798:471–481
Kulma M, Kwiatkowska K, Sobota A (2012) Raft coalescence and FcgammaRIIA activation upon sphingomyelin clustering induced by lysenin. Cell Signal 24:1641–1647
Kuzmin PI, Akimov SA, Chizmadzhev YA, Zimmerberg J, Cohen FS (2005) Line tension and interaction energies of membrane rafts calculated from lipid splay and tilt. Biophys J 88:1120–1133
Masin J, Roderova J, Osickova A, Novak P, Bumba L, Fiser R, Sebo P, Osicka R (2017) The conserved tyrosine residue 940 plays a key structural role in membrane interaction of Bordetella adenylate cyclase toxin. Sci Rep 7:9330
Mate SM, Vazquez RF, Herlax VS, Daza Millone MA, Fanani ML, Maggio B, Vela ME, Bakas LS (2014) Boundary region between coexisting lipid phases as initial binding sites for Escherichia coli alpha-hemolysin: a real-time study. Biochim Biophys Acta 1838:1832–1841
Mesa-Galloso H, Valiente PA, Valdes-Tresanco ME, Epand RF, Lanio ME, Epand RM, Alvarez C, Tieleman DP, Ros U (2019) Membrane remodeling by the lytic fragment of Sticholysin II: implications for the toroidal pore model. Biophys J 117:1563–1576
Mesa-Galloso H, Pedrera L, Ros U (2021) Pore-forming proteins: from defense factors to endogenous executors of cell death. Chem Phys Lipids 234:105026
Metkar SS, Wang B, Catalan E, Anderluh G, Gilbert RJ, Pardo J, Froelich CJ (2011) Perforin rapidly induces plasma membrane phospholipid flip-flop. PLoS One 6:e24286
Moglich A, Yang X, Ayers RA, Moffat K (2010) Structure and function of plant photoreceptors. Annu Rev Plant Biol 61:21–47
Moller N, Ziesemer S, Hildebrandt P, Assenheimer N, Volker U, Hildebrandt JP (2020) S. aureus alpha-toxin monomer binding and heptamer formation in host cell membranes—do they determine sensitivity of airway epithelial cells toward the toxin? PLoS One 15:e0233854
Mondal AK, Chattopadhyay K (2020) Taking toll on membranes: curious cases of bacterial beta-barrel pore-forming toxins. Biochemistry 59:163–170
Mondal AK, Chattopadhyay K (2022) Structures and functions of the membrane-damaging pore-forming proteins. Adv Protein Chem Struct Biol 128:241–288
Mondal AK, Sreekumar A, Kundu N, Kathuria R, Verma P, Gandhi S, Chattopadhyay K (2018) Structural basis and functional implications of the membrane pore-formation mechanisms of bacterial pore-forming toxins. Adv Exp Med Biol 1112:281–291
Mondal AK, Verma P, Lata K, Singh M, Chatterjee S, Chattopadhyay K (2020) Sequence diversity in the pore-forming motifs of the membrane-damaging protein toxins. J Membr Biol 253:469–478
Mulvihill E, van Pee K, Mari SA, Muller DJ, Yildiz O (2015) Directly observing the lipid-dependent self-assembly and pore-forming mechanism of the cytolytic toxin listeriolysin O. Nano Lett 15:6965–6973
Nelson LD, Johnson AE, London E (2008) How interaction of perfringolysin O with membranes is controlled by sterol structure, lipid structure, and physiological low pH: insights into the origin of perfringolysin O-lipid raft interaction. J Biol Chem 283:4632–4642
Nicolson GL (2014) The fluid-mosaic model of membrane structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim Biophys Acta 1838:1451–1466
Ostolaza H, Gonzalez-Bullon D, Uribe KB, Martin C, Amuategi J, Fernandez-Martinez X (2019) Membrane permeabilization by pore-forming RTX toxins: what kind of lesions do these toxins form? Toxins (Basel) 11:354
Parker MW, Feil SC (2005) Pore-forming protein toxins: from structure to function. Prog Biophys Mol Biol 88:91–142
Pedrera L, Gomide AB, Sanchez RE, Ros U, Wilke N, Pazos F, Lanio ME, Itri R, Fanani ML, Alvarez C (2015) The presence of sterols favors Sticholysin I-membrane association and pore formation regardless of their ability to form laterally segregated domains. Langmuir 31:9911–9923
Praper T, Sonnen AF, Kladnik A, Andrighetti AO, Viero G, Morris KJ, Volpi E, Lunelli L, Dalla Serra M, Froelich CJ, Gilbert RJ, Anderluh G (2011) Perforin activity at membranes leads to invaginations and vesicle formation. Proc Natl Acad Sci U S A 108:21016–21021
Puech PH, Borghi N, Karatekin E, Brochard-Wyart F (2003) Line thermodynamics: adsorption at a membrane edge. Phys Rev Lett 90:128304
Pulagam LP, Steinhoff HJ (2013) Acidic pH-induced membrane insertion of colicin A into E. coli natural lipids probed by site-directed spin labeling. J Mol Biol 425:1782–1794
Rai AK, Chattopadhyay K (2015) Revisiting the membrane interaction mechanism of a membrane-damaging beta-barrel pore-forming toxin Vibrio cholerae cytolysin. Mol Microbiol 97:1051–1062
Rai AK, Paul K, Chattopadhyay K (2013) Functional mapping of the lectin activity site on the beta-prism domain of Vibrio cholerae cytolysin: implications for the membrane pore-formation mechanism of the toxin. J Biol Chem 288:1665–1673
Reboul CF, Whisstock JC, Dunstone MA (2016) Giant MACPF/CDC pore forming toxins: a class of their own. Biochim Biophys Acta 1858:475–486
Reddy A, Caler EV, Andrews NW (2001) Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell 106:157–169
Rojko N, Anderluh G (2015) How lipid membranes affect pore forming toxin activity. Acc Chem Res 48:3073–3079
Rojko N, Cronin B, Danial JS, Baker MA, Anderluh G, Wallace MI (2014) Imaging the lipid-phase-dependent pore formation of equinatoxin II in droplet interface bilayers. Biophys J 106:1630–1637
Rojko N, Dalla Serra M, Macek P, Anderluh G (2016) Pore formation by actinoporins, cytolysins from sea anemones. Biochim Biophys Acta 1858:446–456
Romero M, Keyel M, Shi G, Bhattacharjee P, Roth R, Heuser JE, Keyel PA (2017) Intrinsic repair protects cells from pore-forming toxins by microvesicle shedding. Cell Death Differ 24:798–808
Ros U, Garcia-Saez AJ (2015) More than a pore: the interplay of pore-forming proteins and lipid membranes. J Membr Biol 248:545–561
Ros U, Edwards MA, Epand RF, Lanio ME, Schreier S, Yip CM, Alvarez C, Epand RM (2013) The sticholysin family of pore-forming toxins induces the mixing of lipids in membrane domains. Biochim Biophys Acta 1828:2757–2762
Ryzy M, Grabec T, Sedlak P, Veres IA (2018) Influence of grain morphology on ultrasonic wave attenuation in polycrystalline media with statistically equiaxed grains. J Acoust Soc Am 143:219
Sarangi NK, Basu JK (2018) Pathways for creation and annihilation of nanoscale biomembrane domains reveal alpha and beta-toxin nanopore formation processes. Phys Chem Chem Phys 20:29116–29130
Sathyanarayana P, Maurya S, Behera A, Ravichandran M, Visweswariah SS, Ayappa KG, Roy R (2018) Cholesterol promotes cytolysin A activity by stabilizing the intermediates during pore formation. Proc Natl Acad Sci U S A 115:E7323–E7330
Savinov SN, Heuck AP (2017) Interaction of cholesterol with perfringolysin O: what have we learned from functional analysis? Toxins (Basel) 9:381
Scheffer LL, Sreetama SC, Sharma N, Medikayala S, Brown KJ, Defour A, Jaiswal JK (2014) Mechanism of Ca(2)(+)-triggered ESCRT assembly and regulation of cell membrane repair. Nat Commun 5:5646
Seilie ES, Bubeck Wardenburg J (2017) Staphylococcus aureus pore-forming toxins: the interface of pathogen and host complexity. Semin Cell Dev Biol 72:101–116
Sevcsik E, Schutz GJ (2016) With or without rafts? Alternative views on cell membranes. BioEssays 38:129–139
Simons K, Sampaio JL (2011) Membrane organization and lipid rafts. Cold Spring Harb Perspect Biol 3:a004697
Simons K, Vaz WL (2004) Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct 33:269–295
Singer SJ, Nicolson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175:720–731
Sonnen AF, Plitzko JM, Gilbert RJ (2014) Incomplete pneumolysin oligomers form membrane pores. Open Biol 4:140044
Su J, Marrink SJ, Melo MN (2020) Localization preference of antimicrobial peptides on liquid-disordered membrane domains. Front Cell Dev Biol 8:350
Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, Schuchman E, Tabas I, Andrews NW (2010) Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J Cell Biol 189:1027–1038
Tam C, Flannery AR, Andrews N (2013) Live imaging assay for assessing the roles of Ca2+ and sphingomyelinase in the repair of pore-forming toxin wounds. J Vis Exp 78:e50531
Tanaka K, Caaveiro JM, Morante K, Gonzalez-Manas JM, Tsumoto K (2015) Structural basis for self-assembly of a cytolytic pore lined by protein and lipid. Nat Commun 6:6337
Valeva A, Walev I, Gerber A, Klein J, Palmer M, Bhakdi S (2000) Staphylococcal alpha-toxin: repair of a calcium-impermeable pore in the target cell membrane. Mol Microbiol 36:467–476
Verma P, Chattopadhyay K (2021) Current perspective on the membrane-damaging action of thermostable direct hemolysin, an atypical bacterial pore-forming toxin. Front Mol Biosci 8:717147
Verma P, Gandhi S, Lata K, Chattopadhyay K (2021) Pore-forming toxins in infection and immunity. Biochem Soc Trans 49:455–465
Vogele M, Bhaskara RM, Mulvihill E, van Pee K, Yildiz O, Kuhlbrandt W, Muller DJ, Hummer G (2019) Membrane perforation by the pore-forming toxin pneumolysin. Proc Natl Acad Sci U S A 116:13352–13357
von Hoven G, Rivas AJ, Neukirch C, Meyenburg M, Qin Q, Parekh S, Hellmann N, Husmann M (2017) Repair of a bacterial small beta-barrel toxin pore depends on channel width. mBio 8:e02083-16
Wolfmeier H, Schoenauer R, Atanassoff AP, Neill DR, Kadioglu A, Draeger A, Babiychuk EB (2015) Ca(2)(+)-dependent repair of pneumolysin pores: a new paradigm for host cellular defense against bacterial pore-forming toxins. Biochim Biophys Acta 1853:2045–2054
Yamashita D, Sugawara T, Takeshita M, Kaneko J, Kamio Y, Tanaka I, Tanaka Y, Yao M (2014) Molecular basis of transmembrane beta-barrel formation of staphylococcal pore-forming toxins. Nat Commun 5:4897
Yilmaz N, Kobayashi T (2015) Visualization of lipid membrane reorganization induced by a pore-forming toxin using high-speed atomic force microscopy. ACS Nano 9:7960–7967
Yilmaz N, Kobayashi T (2016) Assemblies of pore-forming toxins visualized by atomic force microscopy. Biochim Biophys Acta 1858:500–511
Yilmaz N, Yamaji-Hasegawa A, Hullin-Matsuda F, Kobayashi T (2018) Molecular mechanisms of action of sphingomyelin-specific pore-forming toxin, lysenin. Semin Cell Dev Biol 73:188–198
Zhang Y, Chen X, Gueydan C, Han J (2018) Plasma membrane changes during programmed cell deaths. Cell Res 28:9–21
Acknowledgements
Authors acknowledge Indian Institute of Science Education and Research Mohali for fund support. Authors also acknowledge support from Council of Scientific and Industrial Research (CSIR), India (for research fellowship to K.L.), Department of Biotechnology (DBT), Government of India (for research fellowship to M.S.), and University Grants Commission (UGC), India (for research fellowship to S.C.).
Funding
This study was supported by Indian Institute of Science Education and Research Mohali.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lata, K., Singh, M., Chatterjee, S. et al. Membrane Dynamics and Remodelling in Response to the Action of the Membrane-Damaging Pore-Forming Toxins. J Membrane Biol 255, 161–173 (2022). https://doi.org/10.1007/s00232-022-00227-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-022-00227-z