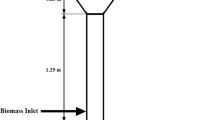
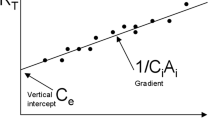
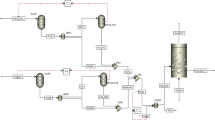
Abstract
A dynamic model has been developed for modeling of carbon dioxide reactive absorption. Mass transfers of the species were considered in both directions. The heat and mass transfer differential equations, were solved using the method of lines. The experiments were carried out to evaluate the model perditions, using an absorption pilot plant. A comparison between the experimental data and the simulation results proves the good predictivity of the presented model.










Similar content being viewed by others
Abbreviations
- a :
-
Specific packing surface (m2/m3)
- A :
-
Cross-sectional area (m2)
- C :
-
Molar concentration (mol/m3)
- C p :
-
Heat capacity (j/m3.k)
- CR :
-
Carbonation ratio (–)
- E :
-
Specific energy holdup (J/m)
- D :
-
Diffusion coefficient (m2/s)
- G :
-
Gas phase molar flow rate (mol/s)
- h :
-
Molar enthalpy (J/mol)
- Δ H :
-
Heat of reaction (J/mol)
- K :
-
Vapor–liquid equilibrium constant (–)
- k :
-
Reaction rate constant (lit/mol.s)
- k L :
-
Mass transfer coefficient (m/s)
- L :
-
Liquid phase molar flow rate (mol/s)
- M :
-
Film conversion parameter (–)
- N :
-
Interfacial molar flux (mol/m2.s)
- q :
-
Heat flux (J/m2.s)
- R :
-
Reaction rate (mol/m3.s)
- T :
-
Temperature (K)
- x :
-
Liquid phase mole fraction (–)
- y :
-
Gas phase mole fraction (–)
- Z :
-
Axial co-ordinate (m)
- δ :
-
Film thickness (m)
- ϕ :
-
Specific molar holdup (mol/m)
- φ :
-
Volumetric holdup (m3/m3)
- λ :
-
Thermal conductivity (W/m.K)
- G :
-
Gas phase
- L :
-
Liquid phase
- i :
-
Component index
- j :
-
Segment index
- s :
-
Component index
- b :
-
Bulk
- f :
-
Film
- g :
-
Gas
- l :
-
Liquid
- I :
-
Interface
References
Kohl A, Nielsen R (1997) Gas purification, 5th edn. Gulf Publishing Company, Houston
Danckwerts PV (1970) Gas-liquid reactions. McGraw-Hill, New York
Taylor R, Krishna R (1993) Multicomponent mass transfer. Wiley, New York
Stankiewicz A, Moulijn J (2004) Re-engineering the chemical processing plant. Marcel Dekker Inc, New York
Sorel E (1893) La rectification de lalcool. Gauthiers-Villaisetfils, Paris
Bradley KJ, Andre H (1972) A dynamic analysis of a packed gas absorber. Can J Chem Eng 50:528–533
Brettschneider O, Thiele R, Faber R, Thielert H, Wozny G (2004) Experimental investigation and simulation of the chemical absorption in a packed column for the system NH3–CO2–H2S–NaOH–H2O. Sep Purif Technol 39:139–159
Kenig EY, Wiesner U, Groak A (1997) Modeling of reactive absorption using the Maxwell Stefan equation. Ind Eng Chem Res 36:4325–4334
Kenig EY, Schneider R, Gorak A (1999) Rigorous dynamic modelling of complex reactive absorption processes. Chem Eng Sci 54:5195–5203
Kenig EY, Kucka L, Gorak A (2003) Rigorous modeling of reactive absorption processes. Chem Eng Technol 26:631–646
Ghaemi A, Shahhosseini Sh, Ghannadi Maragheh M (2009) Nonequilibrium modeling of reactive absorption processes. Chem Eng Commun 196:1076–1089
Ghaemi A, Torab-Mostaedi M, Ghannadi Maragheh M (2011) Kinetics and absorption rate of CO2 into partially carbonated ammonia solutions. Chem Eng Commun 198:1169–1181
Aboudheir A, Tontiwachwuthikul P, Chakma A, Idem R (2003) Kinetics of reactive absorption of carbon dioxide in high CO2-loaded, concentrated aqueous MEA solutions. Chem Eng Sci 58:5195–5210
Yeh AC, Hsunling B (1999) Comparison of ammonia and monoethanolamine solvents to reduce CO2 greenhouse gas emissions. Sci Total Environ 228:121–133
Navaza JM, Gomez D, Dolores M, Rubia L (2009) Removal process of CO2 using MDEA aqueous solutions in a bubble column reactor. Chem Eng J 146:184–188
Rangawala HA, Morrell BR, Mather AE, Otto FD (1992) Absorption of CO2 into aqueous tertiary amine/MEA solutions. Can J Chem Eng 70:482–490
Shen J, Yang Y, Maa J (1999) Promotion mechanism for CO2 absorption into partially carbonated NH3 solutions. J Chem Eng Jpn 32:378–381
Kenig EY, Schneider R, Gorak A (2001) Multicomponent unsteady-state film model: a general analytical solution to the linearized diffusion–reaction problem. Chem Eng J 83:85–94
Krop J (1999) New approach to simplify the equation for the excess gibbs free energy of aqueous solution of electrolytes applied to the modeling of the NH3–CO2–H2O vapor–liquid equilibria. J Fluid Ph Equilib 163:209–229
Zemaitis J, Clark DM, Scrivner NC (1986) Handbook of aqueous electrolyte thermodynamics. AIChE, New York
Billet R, Schultes M (1993) Predicting mass transfer in packed columns. Chem Eng Technol 16:1–9
Reid R, Prausnits J, Poling B (1987) The properties of gases and liquids. McGraw-Hill, New York
Onda K, Takeuch H, Okumoto Y (1968) Transfer coefficient between gas and liquid phase in packed columns. J Eng Jpn 1:56–62
Siddiqi M, Lucas K (1986) Correlations for prediction of diffusion in liquids. Can J Chem Eng 64:839–843
Olanrewaju MJ, Al-Arfaj MA (2005) Development and application of linear process model in estimation and control of reactive distillation. Comput Chem Eng 30:147–157
Coughanowr DR, Koppel LB (1965) Process systems analysis and control. McGraw-Hill, New York
Stephanopoulos G (1984) Chemical process control an introduction to theory and practice. Prentice Hall, Englewood Cliffs, New Jersey
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niknafs, H., Ghaemi, A. & Shahhosseini, S. Dynamic heat and mass transfer modeling and control in carbon dioxide reactive absorption process. Heat Mass Transfer 51, 1131–1140 (2015). https://doi.org/10.1007/s00231-014-1484-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-014-1484-0