Abstract
Purpose
Routine therapeutic drug monitoring of apixaban is currently not recommended but may however be warranted in some situations and for some patient groups to provide better and safer treatment. Due to limited data on apixaban concentrations in different subpopulations, it is still unclear which group of patients could possibly gain from monitoring. The purpose of this study was to examine apixaban exposure in patients with obesity compared with normal-weight patients.
Methods
Forty patients with obesity (mean BMI 39.4 kg/m2) and 40 controls with normal weight (mean BMI 23.4 kg/m2), treated with apixaban 5 mg twice daily were included. The patients were matched for age, sex, and renal function. Trough and peak apixaban concentrations were measured with LC‒MS/MS methodology.
Results
The median trough concentrations in patients with obesity (58.7, range 10.7–200.7 ng/ml) were slightly higher than those in patients with normal weight (52.0, range 31.0–150.9 ng/ml) (p < 0.05). Notably, the variability in trough concentration was considerably higher in patients with obesity. Peak concentrations were similar in both groups, with a median of 124.5 ng/ml (range 82.0–277.5) and 113.5 ng/ml (range 75.5–334.6) in patients with obesity and normal weight, respectively.
Conclusion
Apixaban exposure did not vary substantially between obese and normal weight matched controls, implying that general dose adjustments are not required. However, vast interindividual variability was observed in patients with obesity, suggesting that measuring the concentrations could be valuable for specific patients. Further research is needed to identify which specific patients may benefit from this approach.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity and overweight are a growing global health problem with a prevalence that has almost tripled since the 1970s. In 2016, 39% (1.9 billion) of the adult population were overweight, around one-third of which were obese. A body mass index (BMI) ≥ 25 kg/m2 is classified as overweight, and ≥ 30 kg/m2 is classified as obese [1]. According to the World Health Organization (WHO), obesity and overweight are defined as “abnormal or excessive fat accumulation that presents a risk to health.” Obesity is estimated to be the primary cause of approximately four million deaths each year. With a higher BMI, the risk for several diseases increases, e.g., diabetes, cancer, and cardiovascular diseases, such as atrial fibrillation (AF), atrial flutter (AFL), and venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE) [2,3,4,5]. For the prevention of stroke and systemic embolism in patients with AF and for the treatment/prevention of DVT and PE, oral anticoagulants are used: direct oral anticoagulants (DOACs) or warfarin [6].
The European Society of Cardiology (ESC) recommends using DOACs as first-line therapy instead of warfarin, and the direct factor Xa (FXa) inhibitor apixaban is currently on the top ten list of best-selling drugs globally [7,8,9]. One of the advantages of apixaban and other DOACs is that they, in contrast to warfarin, can be prescribed in a fixed-dose management without the need for routine laboratory monitoring. However, there is an exposure dependency in safety and efficacy, and although routine clinical monitoring is currently not recommended in general for the entire population, in some clinical situations and for some patients, monitoring apixaban concentration could be helpful [10,11,12,13]. Apixaban is a potent drug that can cause serious adverse bleeding if drug exposure is too high but also severe consequences if it is too low, as thrombosis might occur. ISTH SSC guidelines from 2016 suggest that apixaban should not be used in obese with BMI > 40 kg/m2 or weight > 120 kg, and if used, monitoring is recommended [14]. Updated guidelines for VTE were published in 2021 and recommended the use of apixaban in standard doses for the treatment or prevention of VTE in patients with high BMI/weight [15]. However, to our knowledge, this update was based on very limited new published clinical data on patients with obesity. Low body weight is included among the criteria for dose reduction of apixaban, as it may contribute to higher exposure of the drug. Less is known about the opposite, i.e., high body weight, and there are consequently no dose adjustment recommendations provided even though in theory it could mean lower exposure to the drug in this population or in certain individuals [16].
There are scarce data on high body weight in the pivotal clinical trials for apixaban; in some trials, safety/efficacy analyses on patients with high body weight were not presented (AVERROES, AMPLIFY EXT) [17, 18]. However, in patients with acute VTE and a weight ≥ 100 kg or BMI > 35 kg/m2, superior safety was seen for apixaban compared with enoxaparin/warfarin, but no statistically significant difference was seen for the efficacy (AMPLIFY) [19]. A pooled analysis of ADVANCE 2 and 3 showed no difference in either safety or efficacy for patients with BMI ≥ 30 kg/m2 treated with apixaban compared to enoxaparin in the prevention of VTE after orthopedic surgery [20,21,22]. Furthermore, a post hoc analysis of the ARISTOTLE trial (AF patients) showed equal safety and efficacy for apixaban compared with warfarin treatment in patients with weight > 120 kg [23].
In the pivotal studies, the efficacy and safety of apixaban in subpopulations of extreme body weight have thus been presented compared to other means of anticoagulation (i.e., conventional therapy). There is very little knowledge regarding how and whether treatment with apixaban can be optimized at a subpopulation or individual level, as there are limited data on measurements of apixaban plasma concentrations in different sets of patients. The aim of this study was to assess apixaban exposure in patients with obesity compared with normal weight patients with AF/AFL, DVT, or PE.
Methods
This non-interventional prospective study was performed between January 2020 and November 2022. Patients treated with apixaban 5 mg twice daily due to AF/AFL, DVT, or PE according to current clinical recommendations and the summary of product characteristics for Eliquis were recruited from the cardiology department at Danderyd Hospital. Patients with the following criteria were excluded: relative estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73 m2, bariatric surgery, concomitant treatment with strong/moderate CYP3A4 and P-gP inhibitors based on the recommendations in Stockley’s Drug Interactions database and Janusmed drug interaction database (previous name SFINX) [24,25,26]. For the calculation of relative eGFR, the revised Lund-Malmö formula (LM-rev) was used [27]. Creatinine, sex, and age were used to generate the body surface-adjusted relative eGFR. In addition to relative eGFR, absolute eGFR was also calculated using patient length and total body weight (TBW) or ideal body weight (IBW) for estimation of body surface area using the DuBois formula [28]. The IBW was calculated according to the Devine formula (1974) for women as 45.5+(0.91*(length−152.4)) and men 50+(0.91*(length−152.4)) [29].
Patients with a BMI > 30 kg/m2 were enrolled in the obese group and 18.5–24.9 kg/m2 in the normal weight group. Information regarding BMI and weight was extracted from the patients’ medical records or obtained directly from the patients themselves via telephone call. A patient visit was booked during which blood sampling and various measurements were performed at steady state after at least 3 days of apixaban treatment of 5 mg twice daily. Blood samples were collected for analysis of apixaban concentration and s-creatinine. Further activated partial thromboplastin time (aPTT), prothrombin time-international normalized ratio (PT-INR), and hemoglobin (Hb) were analyzed (description of the methods, see Supplementary material). The actual weight, length, and waist size were measured during this patient visit. For each patient, age, sex, concomitant drug treatment, diagnoses, and smoking habits were registered. The information was obtained from the patient medical records and during meeting/contact with the patient.
A total of 40 patients with obesity and 40 normal weight patients, matched for age (± 6 years), sex, and relative eGFR (± 15 if > 60 mL/min/1.73 m2 and ± 10 if < 59 mL/min/1.73 m2), were included.
Measurement of apixaban concentration
Blood samples were drawn by direct venipuncture from an antecubital vein and collected into vacuum tubes containing citrate (5/4.5). The blood samples were centrifuged (2000 ⋅ g) at room temperature for 20 min. Plasma was then aliquoted in 250 µl cryotubes and frozen at −80 °C. The frozen samples were sent in a batch to the Clinical Pharmacology unit at the Karolinska University Hospital for measurement of apixaban concentrations using a gold standard LC‒MS/MS method modified from a previously described method [30]. The method is described in Supplementary material.
Statistical analysis
To estimate the relationship between different variables, Pearson’s correlation analysis was used. In addition, a multiple regression analysis was performed. Differences between groups of patients (e.g., obese and normal weight) in apixaban plasma concentrations were evaluated by Wilcoxon signed rank test for related samples. To estimate differences in patient characteristics between the obese and matched normal weight control patients, a paired sample t test was used for normally distributed variables, and for non-normal variables, the Wilcoxon signed rank test was used. For nominal variables, e.g., comorbidities, McNemar’s test was used. Normality distribution was assessed with histograms and the Shapiro‒Wilk or Kolmogorov‒Smirnov tests.
With 40 patients in each group, we have a 90% power to detect a difference of 25 ng/mL between the matched groups with a standard deviation of 31, a significance level of 5%, and a paired t-test, accounting for a 20% dropout rate.
A p value < 0.05 was considered statistically significant. Statistical analysis was performed in IBM SPSS version 25 (IBM Corp, USA).
Results
Cohort characteristics
A total of 80 patients treated with apixaban 5 mg twice daily were included in this paired analysis, forming 40 matched obese and normal weight pairs. A higher percentage of males were included (72.5%, 29 pairs). VTE was the predominant therapeutic indication for apixaban therapy in 61 patients (76%), while 17 patients were prescribed apixaban for AF/AFL (21%) and two patients for a combination of AF/AFL/VTE (3%). The proportion of comorbidities such as diabetes, hypertension, and/or heart failure was higher in patients with obesity with 65% (20% diabetes, 63% hypertension, 10% heart-failure) compared to 35% (10% diabetes, 25% hypertension) in normal weight patients, p < 0.05. Patient characteristics are presented in Table 1. Weight, BMI, and waist size were the only characteristics that differed between the two groups. When using the TBW to calculate the absolute eGFR, the groups were significantly different. While this was expected due to the extreme body weight in the obese group, absolute eGFR IBW showed no difference between the two groups. There were no significant differences between the groups for aPTT, PT-INR, or Hb (data presented in the supplementary material, Table S1).
Apixaban trough concentrations
Blood samples for apixaban trough plasma concentrations were collected in patients with obesity and in patients with normal weight on average 12.6 h (range: 9.8–16.3) and 12.4 h (range: 9.4–14.8), respectively, after the last apixaban dose intake. Patients with obesity had slightly higher median trough apixaban concentrations than patients with normal weight, 58.7 (range 10.7–200.7) compared with 52.0 (31.0–150.9) ng/ml (p < 0.05). The coefficient of variation (CV) calculated as the ratio of the standard deviation to the mean was 56% for the patients with obesity and 42% for the normal weight patients. A more than 18-fold variation (calculated as the ratio between the highest and lowest value) in apixaban trough concentration was observed in the obese group, and the variation within the 10th–90th percentile was 3-fold (39.3–130.4 ng/ml) (Fig. 1). For the control group, the variation in trough concentrations was less pronounced, with a near 5-fold overall variation and a 2-fold variation within the 10th–90th percentile (34.0–83.1 ng/ml).
Apixaban trough concentrations measured in 40 patients with obesity and 40 matched normal weight patients treated with apixaban 5 mg twice daily. The line inside the box plot represents the median, and the top and bottom lines of the box show Q1 (25th percentile) and Q3 (75th percentile). The whiskers represent minimum and maximum values excluding outliers. Mild outliers (circle) are defined as values outside the ranges from 1.5 × interquartile range (IQR) below Q1 or above Q3
There was no statistically significant correlation between trough concentrations and BMI or weight as visualized in Fig. 2. Among the 40 pairs, the trough concentrations were lower in patients with obesity than in normal weight patients in 12 pairs, and vice versa in 28 pairs.
Apixaban peak concentrations
Peak sampling was collected from those who agreed on an additional blood sampling (meaning they had to stay another approximately 3 h for this), giving peak samples from 22 patients with obesity and 22 normal weight patients forming 22 pairs. The peak concentrations were collected on average 3.1 h (range 2.4–4.0) and 3.2 h (range 2.1–3.8) after the last apixaban dose intake in patients with obesity and normal weight patients, respectively. The obese had similar median peak concentrations as the normal weights (124.5, range 82.0–277.5 ng/ml, CV 39% in obese and 113.5, range 75.5–334.6 ng/ml, CV 43% in normal weights) (Fig. 3).
Apixaban peak concentrations measured with LC‒MS/MS methodology in 22 pairs of patients with obesity and normal weight patients treated with apixaban 5 mg twice daily. The line inside the box plot represents the median, and the top and bottom lines of the box show Q1 (25th percentile) and Q3 (75th percentile). The whiskers represent minimum and maximum values excluding outliers. Mild outliers are defined as values outside the ranges from 1.5 × interquartile range (IQR) below Q1 or above Q3. Extreme outliers (star) are defined as values outside the ranges from 3 × IQR below Q1 or above Q3
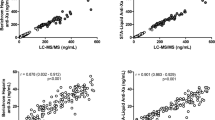
There were no significant correlations between peak concentrations and body weight/BMI (Fig. 4). A strong and significant correlation between trough and peak concentrations was observed; higher trough concentrations were correlated with higher peak concentrations (Fig. 5).
Renal function
There were significant correlations between trough concentrations and renal function as measured by relative eGFR (Fig. 6) and absolute eGFR IBW (data not shown).
Multiple regression analysis
The two groups (patients with obesity and normal weight patients) were individually matched for relative eGFR, age, and sex. Consequently, we conducted separate multiple regression analysis for each group. The multiple regression analysis was performed between trough concentration and body weight or BMI and relative eGFR and sex. For BMI and body weight, the results were non-significant. However, significant results were observed for relative eGFR for the analysis including BMI/body weight. For the BMI analysis, the following numbers were observed for relative eGFR: for the patients with obesity −2.12 (−3.16 to −1.08), p < 0.001 and for the normal weight patients −0.85 (−1.62 to −0.07), p = 0.033. Further, significant results were observed for patients’ sex but only for the patients with normal weight, and for the analysis including BMI, the numbers for female sex were 18.24 (0.84 to 35.64), p = 0.04).
Outliers
Mild outliers are defined as values outside the ranges from 1.5 × interquartile range (IQR) below Q1 or above Q3. Extreme outliers (star) are defined as values outside the ranges from 3 × IQR below Q1 or above Q3. Six patients were defined as outliers at trough and/or peak, three obese and three normal weight. The three patients with obesity were defined as outliers at both trough and peak with trough concentrations between 171.7 and 200.7 ng/ml and peak concentrations between 243.2 and 277.5 ng/ml (Figs. 1 and 3). They were > 70 years old, had a BMI between 38 and 48 kg/m2, and two of the patients had a concurrent diagnosis of heart failure. Their relative eGFR was between 60 and 70 ml/min/1.73 m2 (Fig. 6). One normal weight patient was defined as an outlier at both trough and peak, at peak as an extreme outlier with a concentration of 334.6 ng/ml, and at trough a concentration of 150.9 ng/ml. In addition, one normal weight patient was defined as an outlier at peak (238.0 ng/ml) only and one patient at trough only (125.1 ng/ml) (Figs. 1 and 3). These three normal weight patients were between 45 and 80 years old, and none of them was diagnosed with heart failure. All outliers were included in the analyses.
Subgroup analysis by patient’s sex
An exploratory subgroup analysis by patient’s sex was performed. Similar to the overall results, trough concentrations were, if anything, marginally higher in patients with obesity, both among female patients (median: 67.8 vs. 66.3 ng/ml, patients with obesity and normal weight respectively, n = 22 in total) and male patients (median: 57.4 vs. 49.1 ng/ml patients with obesity and normal weight respectively, n = 58 in total).
Discussion
In our study of 40 obese-normal weighted matched pairs, weight or BMI did not correlate with either trough or peak concentrations. The patients with obesity had if anything slightly higher trough and peak concentrations, although for the peak concentrations, this was not significant. We found thus no evidence that patients with obesity in general are at risk of having lower exposure, i.e., concentration of apixaban in plasma, and therefore in need of higher doses of apixaban than patients of normal weight. Any differences that we found here between patients with obesity and normal weight patients were modest if any, and thus probably not of clinical relevance. The results from our study are consistent with other studies, in which apixaban concentrations were either decreased moderately or with no detected difference in apixaban exposure in obese subjects [31,32,33,34]. These studies were however either quite small and/or lacked a control group. The majority of the concentrations were within the reference range, which was determined based on apixaban concentrations measured in other studies. In one study [31], no relationship between apixaban concentrations and BMI or body weight was observed; however, in another study [34], whereas BMI did not correlate with trough or peak concentrations, weight had a moderate/weak negative correlation. In the abovementioned studies, the drug concentrations measured in obese were compared with apixaban concentrations from other studies. A strength of our study is that we compared the concentrations in obese with a control group constituted by matched normal-weight patients. The matched obese and normal-weight patients were thus similar for other known variables that could potentially affect the concentrations of apixaban. In contrast to the above, we found one study showing that healthy subjects with a body weight ≥ 120 kg and BMI ≥ 30 kg/m2 (n = 19) had 30% lower peak apixaban concentrations compared to normal weights (n = 16) [35]. This was, however, a single-dose study, and the results may not be transferable to patients receiving repeated doses.
The patients with obesity in our study exhibited a larger interindividual variation in trough concentration than the normal weight patients, more than 18-fold (range 11–201) compared with 5-fold (range 31–151). In our previous study in AF patients treated with apixaban 5 mg bid, a 6-fold variation was seen in trough concentration (range 29–186), again much lower variation than for the patients with obesity [30]. The observed larger variation in patients with obesity may indicate that this group has more unpredictable pharmacokinetics of apixaban. As exposure in obese seems less predictable, monitoring of apixaban concentrations could thus be considered. Especially if these patients have other factors that also may influence apixaban concentrations, e.g., medication with interacting drugs or renal insufficiency. An interesting observation from our study was that all three outliers in the obese group had relative eGFR below 70 ml/min/1.73 m2, and two of them had heart failure, indicating that these may be a subpopulation(s) within the obese at risk for very high apixaban exposure and where therapeutic drug monitoring is warranted. This is obviously based on very few observations and needs to be confirmed in a larger population. Another possible explanation for the large variability could be related to apixaban metabolism. Approximately 25% of apixaban is metabolized, mainly via CYP3A4 and to a lesser extent via other CYP450 enzymes [16]. Patients with obesity have been shown to have altered enzyme activity with a negative correlation between BMI and CYP3A4 activity. With weight loss, the activity of CYP3A4 was restored [36]. There are no data on apixaban exposure related to CYP3A4 activity in patients with obesity; thus, the clinical importance of this is uncertain. This is a field that is not fully studied, but one could speculate that the many physiological changes related to obesity may in turn relate to drug exposure variability. Obesity has been associated with different pharmacokinetic alterations not only for metabolism but also for drug absorption with accelerated gastric emptying and gastrointestinal transit, and for drug distribution with increased volume of distribution [37]. A population pharmacokinetic study of apixaban in AF patients showed that a body weight of 90 kg resulted in 22% increase in the volume of distribution compared to a 70-kg subject [38]. Furthermore, obesity is associated with other comorbidities that may give a higher variability between patients. In our study, the normal-weight patients were healthier, as the proportion of obese having other comorbidities was higher than the normal weight patients (65% compared with 35%). In addition, obese have a 3.5-fold increased risk of developing non-alcoholic fatty liver disease (NAFLD), which includes different liver conditions from liver steatosis to liver cirrhosis. In this study, we did not measure any liver function test other than PT-INR, which may be considered a limitation, as liver function impairment could impact apixaban metabolism and thus plasma concentrations [39].
Conclusion
In summary, this study observed similar median apixaban plasma concentrations in patients with obesity and their sex-, age-, and renal function–matched controls with normal weight/BMI. However, the variability in plasma concentrations was considerably higher among the obese, and since it could be of importance to identify individuals with very high/low exposure, monitoring of apixaban plasma concentrations after initiation may have clinical relevance in this group.
Data availability
All available data are included in this published article (and its supplementary material).
References
World Health Organization (2019) Obesity and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed 15 Sept 2023
World Health Organization (2019) Obesity. https://www.who.int/health-topics/obesity#tab=tab_1. Accessed 15 Sept 2023
Abed HS, Wittert GA (2013) Obesity and atrial fibrillation. Obes Rev 14(11):929–938. https://doi.org/10.1111/obr.12056
Frost L, Hune LJ, Vestergaard P (2005) Overweight and obesity as risk factors for atrial fibrillation or flutter: the danish diet, cancer, and health study. Am J Med 118(5):489–495. https://doi.org/10.1016/j.amjmed.2005.01.031
Stein PD, Beemath A, Olson RE (2005) Obesity as a risk factor in venous thromboembolism. Am J Med 118(9):978–980. https://doi.org/10.1016/j.amjmed.2005.03.012
Altiok E, Marx N (2018) Oral anticoagulation. Dtsch Arztebl Int 115(46):776–783. https://doi.org/10.3238/arztebl.2018.0776
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, Group ESCSD (2021) 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 42(5):373–498. https://doi.org/10.1093/eurheartj/ehaa612
Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jimenez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ni Ainle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL, Group ESCSD (2020) 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 41(4):543–603. https://doi.org/10.1093/eurheartj/ehz405
Drug Discovery & Development (2023) The 50 best-selling pharmaceuticals of 2022. https://www.drugdiscoverytrends.com/50-of-2022s-best-selling-pharmaceuticals/. Accessed 1 Oct 2023
Reilly PA et al (2014) The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in Atrial Fibrillation patients: the RE-LY trial (randomized evaluation of long-term anticoagulation therapy). J Am Coll Cardiol 63(4):321–328
Cohen D (2014) Dabigatran: how the drug company withheld important analyses. BMJ 349:g4670
Ruff CT et al (2015) Association between edoxaban dose, concentration, anti-factor xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet 385(9984):2288–2295
FDA. Center for drug evaluation and research. Application number: 202155Orig1s000. Medical review (s). Apixaban clinical review addendum. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202155orig1s000medr.pdf. Accessed 11 Dec 2023
Martin K, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S (2016) Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost 14(6):1308–1313. https://doi.org/10.1111/jth.13323
Martin KA, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S (2021) Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC subcommittee on control of anticoagulation. J Thromb Haemost 19(8):1874–1882. https://doi.org/10.1111/jth.15358
Summary of product characteristics Eliquis (apixaban). Bristol-Myers Squibb/Pfizer. European medicines agency. https://www.ema.europa.eu/. Accessed 1 Sept 2023
Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O’Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S, Committee AS, Investigators (2011) Apixaban in patients with atrial fibrillation. N Engl J Med 364(9):806–817. https://doi.org/10.1056/NEJMoa1007432
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Porcari A, Raskob GE, Weitz JI, Investigators A-E (2013) Apixaban for extended treatment of venous thromboembolism. N Engl J Med 368(8):699–708. https://doi.org/10.1056/NEJMoa1207541
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Masiukiewicz U, Pak R, Thompson J, Raskob GE, Weitz JI, Investigators A (2013) Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 369(9):799–808. https://doi.org/10.1056/NEJMoa1302507
Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM, Investigators A (2010) Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 363(26):2487–2498. https://doi.org/10.1056/NEJMoa1006885
Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P, Investigators A (2010) Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 375(9717):807–815. https://doi.org/10.1016/S0140-6736(09)62125-5
Pineo GF, Gallus AS, Raskob GE, Chen D, Ramirez LM, Ramacciotti E, Lassen MR, Wang L (2013) Apixaban after hip or knee arthroplasty versus enoxaparin: efficacy and safety in key clinical subgroups. J Thromb Haemost 11(3):444–451. https://doi.org/10.1111/jth.12109
Hohnloser SH, Fudim M, Alexander JH, Wojdyla DM, Ezekowitz JA, Hanna M, Atar D, Hijazi Z, Bahit MC, Al-Khatib SM, Lopez-Sendon JL, Wallentin L, Granger CB, Lopes RD (2019) Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and extremes in body weight. Circulation 139(20):2292–2300. https://doi.org/10.1161/CIRCULATIONAHA.118.037955
Stockley’s Drug Interaction Checker. Pharmaceutical press. Medicines complete. https://www.medicinescomplete.com/#/interactions/stockley
Janusmed Interaction Database. Region Stockholm. https://janusmed.se/interaktioner
Bottiger Y, Laine K, Andersson ML, Korhonen T, Molin B, Ovesjo ML, Tirkkonen T, Rane A, Gustafsson LL, Eiermann B (2009) SFINX-a drug-drug interaction database designed for clinical decision support systems. Eur J Clin Pharmacol 65(6):627–633. https://doi.org/10.1007/s00228-008-0612-5
Bjork J, Grubb A, Sterner G, Nyman U (2011) Revised equations for estimating glomerular filtration rate based on the lund-malmo study cohort. Scand J Clin Lab Invest 71(3):232–239. https://doi.org/10.3109/00365513.2011.557086
DuBois D (1916) A formula to estimate the approximate surface area if height and weight be known. Arch Intern Medicine 17:863–871. https://doi.org/10.1001/archinte.1916.00080130010002
Devine BJ (1974) Gentamicin therapy. Drug Intell Clin Pharm 8:650–655
Skeppholm M, Al-Aieshy F, Berndtsson M, Al-Khalili F, Ronquist-Nii Y, Soderblom L, Ostlund AY, Pohanka A, Antovic J, Malmstrom RE (2015) Clinical evaluation of laboratory methods to monitor apixaban treatment in patients with atrial fibrillation. Thromb Res 136(1):148–153. https://doi.org/10.1016/j.thromres.2015.04.030
Martin AC, Thomas W, Mahir Z, Crowley MP, Dowling T, Breen K, Collings V, Moore GW, MacDonald S, Hunt BJ, Cohen AT (2021) Direct oral anticoagulant concentrations in obese and high body weight patients: a cohort study. Thromb Haemost 121(2):224–233. https://doi.org/10.1055/s-0040-1715834
Russo V, Cattaneo D, Giannetti L, Bottino R, Laezza N, Atripaldi U, Clementi E (2021) Pharmacokinetics of Direct Oral Anticoagulants in Patients With Atrial Fibrillation and Extreme Obesity. Clin Ther 43(9):e255–e263. https://doi.org/10.1016/j.clinthera.2021.07.003
Piran S, Traquair H, Chan N, Bhagirath V, Schulman S (2018) Peak plasma concentration of direct oral anticoagulants in obese patients weighing over 120 kilograms: a retrospective study. Res Pract Thromb Haemost 2(4):684–688. https://doi.org/10.1002/rth2.12146
Harkness W, Pipitone O, Joss J, Schiedler M, Shagavah S, Moore R, Hsing J (2022) Observed apixaban anti-xa levels in obese patients. Ann Pharmacother 56(11):1215–1221. https://doi.org/10.1177/10600280221077158
Upreti VV, Wang J, Barrett YC, Byon W, Boyd RA, Pursley J, LaCreta FP, Frost CE (2013) Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol 76(6):908–916. https://doi.org/10.1111/bcp.12114
Rodriguez-Morato J, Goday A, Langohr K, Pujadas M, Civit E, Perez-Mana C, Papaseit E, Ramon JM, Benaiges D, Castaner O, Farre M, de la Torre R (2019) Short- and medium-term impact of bariatric surgery on the activities of CYP2D6, CYP3A4, CYP2C9, and CYP1A2 in morbid obesity. Sci Rep 9(1):20405. https://doi.org/10.1038/s41598-019-57002-9
Gouju J, Legeay S (2023) Pharmacokinetics of obese adults: not only an increase in weight. Biomed Pharmacother 166:115281. https://doi.org/10.1016/j.biopha.2023.115281
Cirincione B et al (2018) Population pharmacokinetics of apixaban in subjects with nonvalvular atrial fibrillation. CPT Pharmacometrics Syst Pharmacol 7(11):728–738
Li L, Liu DW, Yan HY, Wang ZY, Zhao SH, Wang B (2016) Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev 17(6):510–519. https://doi.org/10.1111/obr.12407
Acknowledgements
The authors are grateful to the patients who participated in this study. Thanks to research nurse Maja Månsson for all the help with patient sampling, Gry Öyerhavn for analyzing apixaban concentrations, and Staffan Rosenborg for guidance in renal function measurements.
Funding
Open access funding provided by Karolinska Institute. The study was supported by grants from the Swedish Heart-Lung Foundation, the Stockholm County Council (ALF project), and the Swedish Society of Medicine. Open-access funding was provided by Karolinska Institutet.
Author information
Authors and Affiliations
Contributions
FA, RM, and MS contributed to the conception and design of the study. FA and MS were responsible for patient recruitment. FA performed the statistical analysis and wrote the first draft of the manuscript. JF wrote sections of the manuscript. All authors reviewed, edited, and approved the published manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in accordance with the principles of the Declaration of Helsinki and approved by the Swedish ethical review authority in Stockholm, Sweden (Dnr: 2019-03169).
Consent to participate
Before inclusion, oral and written informed consent was obtained. The study patients were informed that their participation is voluntary and that they at any time without explanation can cancel their participation without affecting their therapy.
Consent for publication
The study patients were informed that a compilation of data would be published in a medical journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Aieshy, F., Skeppholm, M., Fyrestam, J. et al. Apixaban plasma concentrations in patients with obesity. Eur J Clin Pharmacol (2024). https://doi.org/10.1007/s00228-024-03696-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00228-024-03696-4