Abstract
Purpose
To analyze the risk factors influencing the development of cefoperazone-induced coagulopathy in critically ill patients and determine the threshold of serum trough concentration.
Methods
A retrospective case-control study was conducted in the intensive care unit patients treated with cefoperazone, and it was approved by the Ethical Committee of Drum Tower Hospital affiliated with the Medical School of Nanjing University (NO.2023-158-01). Patients were divided into the normal group and coagulopathy group based on prothrombin time. The clinical characteristics of the two groups were compared using univariate analysis. The serum concentration threshold and influencing factors of cefoperazone-induced coagulopathy in critically ill patients were analyzed using the receiver operating characteristic curve and multivariate logistic regression analysis.
Results
A total of 113 patients were included, and cefoperazone-induced coagulopathy occurred in 39 patients, with an incidence of 34.5%. These patients experienced significant prothrombin time prolongation around day 6 (median) after cefoperazone application. The serum trough concentration threshold of cefoperazone-induced coagulopathy in critically ill patients was 87.765 mg/l. Multivariate logistic regression analysis revealed that the APACHE II score (p = 0.034), prophylactic use of vitamin K1 (p < 0.001), hepatic impairment (p = 0.014), and Cmin ≥ 87.765 mg/l (p = 0.005) were associated with cefoperazone-induced coagulopathy.
Conclusion
Cefoperazone-induced coagulopathy usually occurs on the 6th day of cefoperazone use in critically ill patients. The risk will increase in patients with an APACHE II score > 25, hepatic impairment, and cefoperazone Cmin ≥ 87.765 mg/l. Vitamin K1 is effective in preventing this adverse reaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cefoperazone (CPZ) is a third-generation cephalosporin antibiotic that inhibits cell wall peptidoglycan synthesis by blocking the synthesis of peptidoglycan. This results in bacterial cell wall deficiency, leading to bacterial cell expansion and lysis. CPZ has a broad antibacterial spectrum with antibacterial activity against both Gram-positive and negative bacteria. However, the emergence of extended-spectrum β-lactamases (ESBLs) has limited its clinical application. Sulbactam (SAM) is an irreversible competitive β-lactamase inhibitor that protects β-lactam antibiotics from hydrolytic destruction. It has a bactericidal effect on gonococci and Fusobacterium spp. when used alone [1]. Cefoperazone sodium and sulbactam sodium for injection (CPZ/SAM) is a combination formulation that was introduced to the Chinese market in 1997. The combination of the two drugs had a significant synergistic effect, with a fourfold higher antibacterial effect than that of using CPZ alone [2]. It can be used to treat infections of the respiratory, digestive, and urinary tracts caused by common pathogens such as Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia [3]. Due to its significant therapeutic effect and high safety profile, CPZ/SAM has been recommended as the first choice empirical treatment for various infectious diseases in China. However, with the widespread use of CPZ/SAM, the incidence of adverse reactions has increased. Since the 1980s, adverse events related to coagulation dysfunction caused by CPZ have been reported several times, and case reports of blood in urine, abdominal wall hematoma [4], and upper gastrointestinal bleeding [5] caused by CPZ have received widespread attention. Until 2019, NMPA has added the content of adverse reactions such as “coagulation disorders, bleeding” to the instructions of drugs containing CPZ.
Nevertheless, the threshold of CPZ exposure toxicity, especially the upper limit of serum concentration for CPZ-induced coagulation disorders, is unknown. Therefore, this study aimed to determine the serum concentration thresholds and risk factors for CPZ-induced coagulopathy in critically ill patients to reduce the incidence of bleeding events and mortality and to ensure patient safety with the drug.
Materials and methods
Study design
This was a single-center, retrospective, case-control study of critically ill patients from May 2021 and May 2023 at the intensive care unit of Drum Tower Hospital affiliated with the Medical School of Nanjing University [6]. All patients received CPZ/SAM (Sulperazon; Pfizer Inc, Shanghai, China, CPZ: SAM = 2:1) at a dosage of 3 g every 8 or 12 h. The dosage and prophylactic use of vitamin K1 were determined by the physician based on the patient’s pathophysiological condition. The inclusion criteria included patients ≥ 18 years of age and use of CPZ/SAM > 5 doses with monitoring of CPZ serum trough concentrations and monitoring of coagulation both before and after administration of the drug. Pregnant and lactating women, patients on anticoagulation therapy with warfarin, and patients with incomplete clinical records or relevant test data were excluded from the study.
Data collection and definitions
Demographic and clinical data of patients meet the inclusion criteria through the hospital information system, including age, gender, diagnosis, acute physiology and chronic health evaluation (APACHE II), sequential organ failure assessment (SOFA), Nutric score, feeding status (general diet, liquid or semi-fluid, fasting), comorbidities (hypertension, diabetes, malignant tumors, cerebrovascular disease, hepatic impairment, renal disease, hematologic disease, autoimmune disease), surgery, continuous renal replacement therapy (CRRT), site of infection, bacterial type, dosage and duration of treatment, and serum trough concentration (Cmin) of CPZ. If the Cmin is monitored several times during treatment, the maximum value will be taken. Laboratory indicators before CPZ medication including infection indicators, coagulation, liver function, renal function indicators, and other drugs used in combination that affect coagulation were obtained. Remedial measures should be taken according to the patient’s bleeding and coagulation function test results, including discontinuing medication and supplementing with vitamin K1, plasma, or other coagulation factors (cold precipitation, prothrombin complex). The coagulation function should be rechecked until it returns to normal.
An increased prothrombin time (PT) of 25% from baseline is defined as coagulopathy. Bleeding events are defined as having a bleeding-related diagnosis at discharge, but not at admission. Patients with adverse events were scored using the Naranjo scoring system to analyze the association between CPZ and coagulopathy. This study defines at least “possible” as CPZ-induced coagulopathy. Each case involving a suspected adverse event was independently reviewed by two researchers (one clinical pharmacist and one physician) for analysis.
Measurement of CPZ serum concentration
After receiving at least 5 doses of CPZ therapy, the patient’s blood sample of 3 ml was collected before the next administration as Cmin. The sample was placed in an inert separation gel procoagulant vacuum blood collection tube, shaken, and centrifuged at 3000 r/min for 10 min, and the upper clear liquid was frozen and stored at −20 °C until analysis. If the patient adjusts the dosage, then the above method should be used to collect blood samples for measuring the Cmin. The determination of Cmin is performed by validated liquid chromatography-tandem mass spectrometry (UPLC I-Class/Xevo TQD IVD system, Waters, USA), with an Acquity UPLC®BEH C18 1.7 μm IVD column (2.1 mm × 50 mm) as the chromatographic column. The mobile phase consists of (a) aqueous solution of formic acid and (b) methanol solution of formic acid, with gradient elution at a flow rate of 0.5 ml/min and a column temperature of 45 °C. The gradient program was as follows: 0–0.6 min, 95% A; 0.6–1.4 min, 95–20% A; 1.4–1.7 min, 20–5% A; 1.7–2.0 min, 5–95% A. Mass spectrometry is performed using an electrospray ionization source. The linear range of CPZ concentration was 0.2–20 mg/l, and the lower limit of quantification (LLOQ) is 0.02 mg/l, with precision and accuracy both within 15%.
Statistical analyses
IBM SPSS Statistics, version 26.0 (IBM Corp., Armonk, N.Y., USA) and GraphPad Prism, version 8.0 (GraphPad Software, San Diego, California USA) were applied for analysis and graphing. For continuous variables, conformity to a normal distribution was expressed as mean ± standard deviation (\(\overline{X }\pm S\)), and continuous non-normally distributed measures were expressed as median (M) and interquartile range (IQR). Data were compared between two groups using either the t test (normal distribution) or the Mann–Whitney U test (non-normal distribution). Categorical variables were expressed as number (%), and comparisons between two groups were made using the Pearson chi-square test, continuity correction test, or Fisher exact test. Factors with p < 0.1 in the baseline variables analysis were included in the forward multivariate logistic regression analysis, and odds ratios (OR) and 95% confidence intervals (CIs) were calculated. A p-value of < 0.05 indicated statistical significance. The receiver operating characteristic (ROC) curve was used to determine the threshold of Cmin of CPZ that causes coagulation disorders in critically ill patients.
Results
Demographics and clinical characteristics
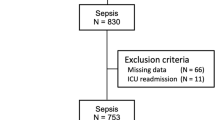
From May 2021 to April 2023, a total of 125 critically ill patients in our ICU met the inclusion criteria. Among them, 12 patients were excluded based on exclusion criteria, including 8 patients who received less than 5 doses of CPZ/SAM, 3 patients who did not measure Cmin, and 1 patient who did not have coagulation indicators monitored after medication. Ultimately, a retrospective analysis was conducted on 113 patients who were included (Fig. 1). The predominant population of patients was male (67.3%), with an average age of 68.05 ± 17.23 years. In addition to empirical medication (45.1%), the primary site of infection was the lungs (35.4%). In infections confirmed by microbiology, the primary pathogen was Acinetobacter baumannii (25.7%), and patients infected with two or more pathogens account for 14.2%. A total of 39 patients (coagulopathy group) developed coagulation disorders after receiving treatment with CPZ/SAM, while 74 patients did not develop coagulation disorders (normal group), resulting in a coagulation disorder incidence rate of 34.5%. The baseline characteristics of the two groups of patients are shown in Table 1, including gender, age, SOFA score, infection site, pathogenic microorganisms, dosage, treatment duration, and eating status, which were not statistically different (p > 0.05).
Effect of CPZ on coagulation and clinical outcomes
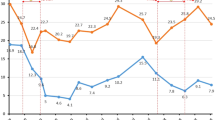
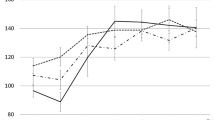
Two groups of patients’ coagulation indexes before and after CPZ treatment are shown in Table 2. There was no significant difference in PT between the two groups at baseline (p = 0.690). However, after CPZ treatment, the PT of patients with coagulopathy was significantly prolonged to 19.9 (16.7–27.3) seconds compared to 12.6 (11.7–14.5) seconds before medication (p = 0.042). After administering vitamin K1 or other blood products, the PT level was significantly reduced (p < 0.05), as shown in Fig. 2. The Kaplan-Meier curve shows that the median time from the start of treatment to the induction of coagulation disorders by CPZ is 6 days (Fig. 3). As the duration of use increased, the number of patients with abnormal coagulation function gradually increased. After 14 days of CPZ treatment, 32.74% of patients experienced coagulation disorders.
Among patients with coagulation dysfunction, a total of 8 patients experienced bleeding of varying degrees, including 5 with gastrointestinal bleeding, 1 with diffuse alveolar hemorrhage, and 1 with local bleeding of the right iliac muscle. The incidence of bleeding was significantly different compared to the normal group (p = 0.001). In terms of blood product infusion, patients with coagulopathy took corresponding remedial measures after experiencing adverse reactions, and the infusion frequency was significantly higher than that of the normal group (p < 0.001). Compared with the normal group, the ICU hospitalization time (p = 0.454), recovering rate (p = 0.301), and automatic discharge rate (p = 0.243) of the coagulopathy group were not statistically significant, but the 28-day all-cause mortality was significantly higher than that of the normal group (p = 0.01) (Table 3).
Risk factors for CPZ-associated coagulopathy
In the analysis of baseline data of patients, the development of coagulopathy caused by CPZ was related to APACHE II score (p = 0.063), vitamin K1 prevention (p < 0.001), concurrent liver dysfunction (p < 0.001), Nutric score (p = 0.052), combined use of tigecycline (p = 0.041), gabapentin mesylate (p = 0.071), IL-6 level (p = 0.027), CRP level (p = 0.09), and creatinine clearance rate (CLcr) (p = 0.093). In the study, the Cmin of the normal group and the coagulopathy group were found to be 73.085 (35.75–133.73) mg/l and 137.6 (73.3–180.8) mg/l, respectively. The difference between the two groups was statistically significant (p = 0.003). Furthermore, based on the ROC curve (Fig. 4), the Cmin cut-off value associated with an increased risk of coagulation disorders was found to be 87.765 mg/l. The area under the ROC curve (AUC) was calculated to be 0.734, with a sensitivity of 71.8% and a specificity of 70.3%. Variables with a p-value < 0.1 were included in a multiple forward logistic regression analysis (Table 4). The results indicated that upon admission to the ICU, the APACHE II score (OR, 3.229; p = 0.034), prophylactic use of vitamin K1 (OR, 0.08; p < 0.001), co-existing hepatic impairment (OR, 5.616; p = 0.014), and Cmin ≥ 87.765 mg/l (OR, 5.045; p = 0.005) are independent factors affecting the occurrence of CPZ-induced coagulopathy in patients.
Discussion
Coagulopathy is a more serious adverse reaction during the use of CPZ-containing drugs, and the incidence of coagulation dysfunction in patients using CPZ/SAM has been reported to be about 25.8% or even up to 45% in clinical practice [7]. The main manifestations are reduced prothrombin activity, prolonged prothrombin time, and thrombocytopenia [8, 9]. Grasela et al. [10] concluded that patients with severe disease are at the highest risk of developing coagulopathy. Due to their specific pathophysiological situation, critically ill patients may already be in a relatively disturbed state of coagulation. This phenomenon is mainly associated with endothelial cell damage caused by factors such as the release of inflammatory transmitters and activation of leukocyte adhesion factors in patients, or with the inflammatory response of patients [11]. At the same time, the pharmacokinetics of CPZ in critically ill patients undergo significant changes [12], which increases the risk of CPZ-induced coagulation dysfunction. Patients with severe coagulopathy not only experience a significant increase in bleeding events and transfusion volume but are also more likely to develop multiple organ dysfunction syndrome, with a mortality rate more than 4 times higher than that of patients with normal coagulation function [13]. This study showed that the incidence of coagulation dysfunction induced by CPZ in critically ill patients is 34.5%, with a bleeding event rate of 7.08%. Patients in the coagulopathy group experienced significant PT prolongation around 6 days (median) after CPZ application.
This study attempted to determine the threshold of toxic exposure to CPZ by therapeutic drug monitoring (TDM). To our knowledge, this is the first study on the concentration-response relationship of CPZ-induced coagulation disorders. Previous studies only found that CPZ was associated with an increased risk of hypoprothrombinemia and bleeding, and there was a dose–response relationship. Chen et al. [14] suggested that patients who used anticoagulants, suffered from liver failure, or malnutrition, and had a history of bleeding events faced a significantly increased risk of bleeding events. They calculated the cumulative dose of CPZ based on a defined daily dose (DDD), and when the cumulative dose exceeded 5 DDDs, the incidence of bleeding events was even higher. In a retrospective cohort study involving 476 patients, the risk of prolongation of PT was found to increase fivefold in the high-dose group of CPZ (> 4.5 g/d) compared to the cefotaxime or ceftizoxime group and 16-fold compared to the ceftazidime group [15]. Andrassy et al. [16] also demonstrated that in the presence of high CPZ serum Cmin, a platelet defect with prolonged bleeding time and impaired platelet aggregation may occur. In this study, the Cmin threshold for CPZ-induced coagulation dysfunction was 87.765 mg/l, and the AUC was 0.734, which had a good predictive value by plotting the ROC curve. Moreover, the results of multivariate logistic regression analysis showed that cefepime Cmin ≥ 87.765 mg/l was associated with a fivefold increase in the risk of CPZ-induced coagulopathy in patients.
Vitamin K is an essential cofactor for the carboxylation of hepatic cell mitochondria. It is involved in the γ-carboxylation reaction of glutamic acid in prothrombin precursors and is mainly obtained from daily dietary intake [17]. Many studies have suggested that there are two main mechanisms for CPZ-induced coagulopathy: (1) CPZ is hardly metabolized in the body, with about 75% excreted in bile, inhibiting the growth of normal intestinal flora and reducing the synthesis of vitamin K in the intestines; (2) the N-methylthiotetrazole (NMTT) side chain carried by CPZ has a structure similar to glutamic acid, which can interfere with the carboxylation of vitamin K in the liver, thereby reducing the synthesis of prothrombin and lowering the levels of coagulation factors II, VII, IX, and X that depend on vitamin K [18, 19]. Nevertheless, prophylactic use of vitamin K1 prior to CPZ therapy remains controversial. In a retrospective cohort study of 374 patients treated with CPZ in a teaching hospital, prophylactic use of vitamin K did not reduce bleeding [15]. Rockoff et al. [20] concluded that routine use of vitamin K and CPZ for perioperative infection prophylaxis may not be necessary. The results of a clinical trial showed that 141 patients who received CPZ without prophylactic vitamin K did not bleed [21]. The logistic regression results of this study indicated that the prophylactic application of vitamin K1 is an independent protective factor for preventing CPZ-induced coagulation disorders. Prophylactic administration of vitamin K1 can significantly prevent patients’ coagulation disorders, even when exposed to higher concentrations of CPZ.
Currently, there were many studies on the factors influencing the abnormal coagulation function caused by CPZ, and a large number of studies believed that the risk of this coagulopathy is closely related to the patient’s own pathological and physiological conditions. Advanced age, cancer, malnutrition, underlying hepatic impairment, renal insufficiency, combined use of anticoagulants, and previous bleeding history were all considered risk factors for CPZ-induced coagulopathy [7, 22]. The results of this study showed that combined hepatic impairment was an independent risk factor for coagulation dysfunction in patients (OR, 5.616; p = 0.014), which was consistent with previous studies. Hepatic insufficiency can lead to reduced drug metabolism and accumulation in the body, as well as a decreased ability of the liver to synthesize its own coagulation factors, leading to abnormal coagulation [22]. In a study of 35 patients with severe infections and renal dysfunction [23], in non-jaundiced patients with abnormal liver function, the mean peak and trough serum concentrations produced by a 2-g dose every 12 h were 254 and 125 mg/l, respectively, while in five tests with normal liver function, they were 179.5 and 19.5 mg/l, respectively. Although the trough value increased significantly, there was no statistical significance (p > 0.1).
In this study, an APACHE II score > 25 was also found to be associated with CPZ-induced coagulopathy (OR, 3.229; p = 0.034). The APACHE II score is currently the most widely used system for assessing the condition and prognosis of critically ill patients in clinical settings and can serve as an indicator for evaluating the condition and prognosis of patients in intensive care units [24]. It has been shown that critically ill patients with an APACHE II score of > 10 are at high risk for severe malnutrition [25]. This also explained why patients with higher APACHE II scores are more likely to experience CPZ-related coagulation dysfunction. However, when the Nutric score (p = 0.052) was included in a multivariate logistic regression, it was progressively eliminated from the model. This may be related to the bias in the definition of malnutrition and nutrition scores of the included population. Meanwhile, there was no statistical difference in gender between the two groups (p = 0.052). However, in previous studies, results showed that when including patients under 12 years old, the incidence of antibiotic-associated hypoprothrombinemia in males was higher than in females. They concluded that according to the effect of sex hormones on prothrombin, in the presence of estrogen, prothrombin formation is faster, and the effective concentration of vitamin K is lower. The level of prothrombin is higher in women than in men, and the dietary requirement of vitamin K is lower in women [26]. Perhaps because of differences in the included populations, this study could not reach similar conclusions. Cohort studies with larger sample sizes may be needed regarding the effect of sex hormones on prothrombin.
Drugs that may cause coagulation disorders in patients were also included in the analysis, including antiplatelet agents, other antimicrobials, drugs that may cause bleeding (NSAIDs and glucocorticoids), and anticoagulants (heparin and new oral anticoagulants) [27]. Univariate analysis showed that the combined use of tigecycline was associated with prolonged PT in patients. According to current researches, tigecycline could cause prolongation of PT and APTT, elevation of INR, decreased platelet count [28], and hypofibrinogenemia. Hu et al. [29] analyzed the characteristics of patients with hypofibrinogenemia who received treatment with CPZ/SAM and found that these patients had a higher incidence of coagulation abnormalities (p = 0.009) and required more blood products (p = 0.003). However, this study did not obtain similar results, possibly due to the small sample size and the use of vitamin K1 for prevention, indicating the need for large-scale cohort studies to draw conclusions. Several recent studies have also yielded similar conclusions [30,31,32]. Furthermore, Miao et al. [31] also investigated the effect of the combined use of sodium valproate on CPZ-induced coagulation disorders, and the results showed that sodium valproate is not associated with this adverse reaction, which is consistent with our research findings. Low-dose aspirin is commonly used for antiplatelet therapy, but its antiplatelet mechanism may lead to an increased risk of bleeding. When patients on long-term aspirin use CPZ for antibiotic therapy, the combination of CPZ and aspirin can have a cumulative effect on coagulation dysfunction, resulting in a significant increase in the risk of bleeding [33]. However, this study has not yielded similar conclusions, possibly because the duration of combined use of aspirin and CPZ in ICU patients was insufficient for us to observe the occurrence of this phenomenon. A prospective observational study has also concluded that the combination of vancomycin, a commonly used drug for Gram-positive bacteria in the ICU, with CPZ could lead to a significantly higher risk of coagulation disorders [34]. Although this study did not find any drug combination as a risk factor for CPZ-induced coagulation dysfunction, caution should still be exercised in clinical practice regarding the potential bleeding risk associated with these drugs.
Obviously, the patient’s own risk factors are an important component of adverse reactions, and higher serum Cmin levels of CPZ amplify these inducing factors [35]. This study has certain limitations. Firstly, this is a single-center, retrospective case-control study, which cannot exclude the bias of medical monitoring. Secondly, the heterogeneity of critically ill patients limited our statistical analysis. The serum Cmin threshold of coagulation disorders caused by CPZ needs further multicenter, prospective, randomized controlled studies to determine. Thirdly, this is a study aimed at the Chinese population and does not involve discussion of other races. We did not conduct genetic testing on the patients, and in fact, pharmacogenetics is also an area worth exploring. For example, since CPZ is mainly excreted via bile, it has been suggested that multidrug resistance-associated protein (MRP) 2 (ABCC2) is the transporter protein primarily responsible for CPZ excretion. In patients with hereditary MRP2 (ABCC2) expression deficiency, if there is no compensatory efflux mediated by other transporter proteins, it may lead to obstruction of CPZ excretion in bile, resulting in elevated blood drug concentration and potential occurrence of coagulopathy [36, 37]. Not only that but due to the possibility of vitamin K epoxide reductase (VKOR) being one of the targets of NMTT [18], we are unable to rule out its pharmacogenetic effects. Whether single nucleotide polymorphisms (SNPs) in VKOR increase CPZ sensitivity and lead to coagulation disorders in patients, further genomic research may be needed to confirm this hypothesis. Nevertheless, our study results still provide valuable information about the serum Cmin threshold of coagulopathy caused by CPZ in real-world patients.
In conclusion, we found that the incidence of coagulopathy in critically ill patients treated with CPZ was 34.5%, with a higher likelihood of bleeding events. The median time for the occurrence of coagulation dysfunction was 6 days, and the CPZ serum Cmin threshold was 87.765 mg/l. Prophylactic application of vitamin K1 significantly reduces the incidence of this adverse reaction in critically ill patients. We recommend that critically ill patients with an APACHE II score > 25 and combined hepatic impairment should have timely TDM and coagulation monitoring with the application of CPZ-containing drugs, and it would be more prudent to apply vitamin K1 prophylaxis, while ensuring the efficacy of CPZ treatment in order to prevent coagulopathy and fatal bleeding events.
Data availability
The data used and/or analyzed in this study are available from the corresponding author on reasonable request.
References
Chen R, Ma D, Cai X et al (2022) Quality assessment of domestic cefoperazone sodium and sulbactam sodium for injection. Chinese J Antibiotics 01:55–65. https://doi.org/10.13461/j.cnki.cja.007271
Dai Q, Lv B (2020) Clinical effect of cefoperazone combined with sulbactam in the treatment of patients with pyelonephritis and its influence on liver and kidney function. Guide China Med 29:91–92. https://doi.org/10.15912/j.cnki.gocm.2020.29.043
Du XX, Lv XJ, Qu JY et al (2020) Experts consensus of clinical application of β-lactam antibiotics/β-lactamase inhibitor combination preparations (2020 edition). Natl Med J China 100:738–747. https://doi.org/10.3760/cma.j.cn112137-20200202-00178
Cai Z, Yang W, He Y et al (2016) Cefoperazone/sulbactam-induced abdominal wall hematoma and upper gastrointestinal bleeding: a case report and review of the literature. Drug Saf Case Rep 1:2. https://doi.org/10.1007/s40800-016-0025-9
Katukuri GR, Maddala RN, Ramamoorthi K et al (2016) cefoperazone induced gastrointestinal bleeding. J Clinical Diagnostic Res 8:OD10–OD11. https://doi.org/10.7860/JCDR/2016/19694.8316
von Elm E, Altman DG, Egger M et al (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England) 370:1453–1457. https://doi.org/10.1016/S0140-6736(07)61602-X
Alagozlu H, Cindoruk M, Unal S (2006) Severe INR elevation in a patient with choledocholithiasis receiving cefoperazone. Clin Drug Investig 8:481–484. https://doi.org/10.2165/00044011-200626080-00006
Ling Y, Tan M (2020) Effects of vitamin K1 injection on coagulation function in bacterial infected patients with cefoperazone sulbactam in ICU. Lab Med Clin 16:2347–2349. https://doi.org/10.3969/j.issn.1672-9455.2020.16.022
Park GH, Kim S, Kim MS et al (2019) The association between cephalosporin and hypoprothrombinemia: a systematic review and meta-analysis. Int J Environ Res Public Health 20:3937. https://doi.org/10.3390/ijerph16203937
Grasela TH, Walawander CA, Welage LS et al (1989) Prospective surveillance of antibiotic-associated coagulopathy in 970 patients. Pharmacotherapy 3:158–164. https://doi.org/10.1002/j.1875-9114.1989.tb04122.x
Shenkman B, Budnik I, Einav Y et al (2017) Model of trauma-induced coagulopathy including hemodilution, fibrinolysis, acidosis, and hypothermia: impact on blood coagulation and platelet function. J Trauma Acute Care Surg 2:287–292. https://doi.org/10.1097/TA.0000000000001282
Gao C, Tong J, Yu K et al (2016) Pharmacokinetics of cefoperazone/sulbactam in critically ill patients receiving continuous venovenous hemofiltration. Eur J Clin Pharmacol 7:823–830. https://doi.org/10.1007/s00228-016-2045-x
Stensballe J, Henriksen HH, Johansson PI (2017) Early haemorrhage control and management of trauma-induced coagulopathy: the importance of goal-directed therapy. Curr Opin Crit Care 6:503–510. https://doi.org/10.1097/MCC.0000000000000466
Chen LJ, Hsiao FY, Shen LJ et al (2016) Use of hypoprothrombinemia-inducing cephalosporins and the risk of hemorrhagic events: a nationwide nested case-control study. PLoS ONE 7:e0158407. https://doi.org/10.1371/journal.pone.0158407
Strom BL, Schinnar R, Gibson GA et al (1999) Risk of bleeding and hypoprothrombinaemia associated with NMTT side chain antibiotics: using cefoperazone as a test case. Pharmacoepidemiol Drug Saf 2:81–94. https://doi.org/10.1002/(SICI)1099-1557(199903/04)8:2%3c81::AID-PDS411%3e3.0.CO;2-G
Andrassy K, Koderisch J, Fritz S et al (1986) Alteration of hemostasis associated with cefoperazone treatment. Infection 1:27–31. https://doi.org/10.1007/BF01644806
Chen Y, Shen Z (2003) Haematological adverse effects of cephalosporin. J Clin Intern Med 09:455–457. https://doi.org/10.3969/j.issn.1001-9057.2003.09.003
Shearer MJ, Bechtold H, Andrassy K et al (1988) Mechanism of cephalosporin-induced hypoprothrombinemia: relation to cephalosporin side chain, vitamin K metabolism, and vitamin K status. J Clin Pharmacol 1:88–95. https://doi.org/10.1002/j.1552-4604.1988.tb03106.x
Agnelli G, Del Favero A, Parise P et al (1986) Cephalosporin-induced hypoprothrombinemia: is the N-methylthiotetrazole side chain the culprit? Antimicrob Agents Chemother 6:1108–1109. https://doi.org/10.1128/AAC.29.6.1108
Rockoff SD, Blumenfrucht MJ, Irwin RJ et al (1992) Vitamin K supplementation during prophylactic use of cefoperazone in urologic surgery. Infection 3:146–148. https://doi.org/10.1007/BF01704604
Xin X, Jian L, Xia X et al (2013) A multicentre clinical study on the injection of ceftriaxone/sulbactam compared with cefoperazone/sulbactam in the treatment of respiratory and urinary tract infections. Ann Clin Microbiol Antimicrob 12:38. https://doi.org/10.1186/1476-0711-12-38
Zhu Y, Liang L, Shen J et al (2022) Clinical features and risk factors of coagulation disorders caused by cefoperazone sodium and sulbactam sodium. Chin Pharm J 9(1):741–746. https://doi.org/10.11669/cpj.2022.09.009
Sattler FR, Colao DJ, Caputo GM et al (1986) Cefoperazone for empiric therapy in patients with impaired renal function. Am J Med 2:229–236. https://doi.org/10.1016/0002-9343(86)90256-1
Knaus WA, Draper EA, Wagner DP et al (1985) APACHE II: a severity of disease classification system. Crit Care Med 10:818–829
Kondrup J, Rasmussen HH, Hamberg O et al (2003) Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nut (Edinburgh, Scotland) 3:321–336. https://doi.org/10.1016/s0261-5614(02)00214-5
Aziz F, Patil P (2015) Role of prophylactic vitamin K in preventing antibiotic induced hypoprothrombinemia. Indian J Pediatr 82:363–367. https://doi.org/10.1007/s12098-014-1584-3
Wang W, Liu Y, Yu C et al (2020) Cefoperazone-sulbactam and risk of coagulation disorders or bleeding: a retrospective cohort study. Expert Opin Drug Saf 3:339–347. https://doi.org/10.1080/14740338.2020.1713090
Zhang Q, Zhou S, Zhou J (2015) Tigecycline treatment causes a decrease in fibrinogen levels. Antimicrob Agents Chemother 3:1650–1655. https://doi.org/10.1128/AAC.04305-14
Hu J, Xiao YH, Zheng Y et al (2020) Clinical characteristics and risk factors of tigecycline-associated hypofibrinogenaemia in critically ill patients. Eur J Clin Pharmacol 7:913–922. https://doi.org/10.1007/s00228-020-02860-w
Leng B, Xue YC, Zhang W et al (2019) A retrospective analysis of the effect of tigecycline on coagulation function. Chem Pharm Bull 67(3):258–264. https://doi.org/10.1248/cpb.c18-00844
Miao W, Guo J, Cheng H et al (2023) Risk factors for cefoperazone/sulbactam-induced coagulation disorder. Infect Drug Resist 16:6277–6284. https://doi.org/10.2147/IDR.S429706
Zhang L, Cai X, Peng F et al (2023) Comparison of bleeding risk and hypofibrinogenemia-associated risk factors between tigecycline with cefoperazone/sulbactam therapy and other tigecycline-based combination therapies. Front Pharmacol 14:1182644. https://doi.org/10.3389/fphar.2023.1182644
Wu SX, Wu G, Wu HB (2021) A comparison of coagulation function inpatients receiving aspirin and cefoperazone-sulbactam with and without vitamin K1: a retrospective, observational study. Clin Ther 43:e335–e345. https://doi.org/10.1016/j.clinthera.2021.10.005
Gudivada KK, Krishna B, Sampath S (2023) Cefoperazone-induced coagulopathy in critically ill patients admitted to intensive care unit. Indian journal of critical care medicine 27:183–189. https://doi.org/10.5005/jp-journals-10071-24417
Schentag JJ, Welage LS, Grasela TH et al (1987) Determinants of antibiotic-associated hypoprothrombinemia. Pharmacotherapy 3:80–86. https://doi.org/10.1002/j.1875-9114.1987.tb03522.x
Kato Y, Takahara S, Kato S et al (2008) Involvement of multidrug resistance-associated protein 2 (Abcc2) in molecular weight-dependent biliary excretion of beta-lactam antibiotics. Drug Metab Dispos 36:1088–1096. https://doi.org/10.1124/dmd.107.019125
Keppler D (2011) Multidrug resistance proteins (MRPs, ABCCs): importance for pathophysiology and drug therapy. Handb Exp Pharmacol 201:299–323. https://doi.org/10.1007/978-3-642-14541-4_8
Acknowledgements
The authors would like to thank the staff at the ICU ward and the pharmacy department of Nanjing Drum Tower Hospital. We also acknowledge the support of Huanyan Pan and Zhangyang Lu for their coordination.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by Qian Wang, Pei Liang and Yin Xu. The first draft of the manuscript was written by Qian Wang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethical Committee of Drum Tower Hospital affiliated with the Medical School of Nanjing University (NO.2023-158-01).
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Q., Liang, P., Xu, Y. et al. Serum trough concentration threshold and risk factors of cefoperazone-induced coagulopathy in critically ill patients: A retrospective case-control study. Eur J Clin Pharmacol 80, 737–746 (2024). https://doi.org/10.1007/s00228-024-03634-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-024-03634-4