Abstract
Purpose
Vancomycin is commonly administered as an intermittent infusion (IIV), although vancomycin’s stability at room temperature permits administration continuously over 24 h (CIV). At our institution, CIV has been the preferred infusion method for over 20 years due to ease of administration and simplicity of therapeutic drug monitoring. The purpose of this study was to examine the outcomes associated with IIV compared to CIV.
Methods
This was a retrospective study of patients who received vancomycin for MRSA bacteremia. The primary outcomes were the time to therapeutic goal and frequency of adverse drug reactions on IIV compared to CIV. Secondary outcomes evaluated all-cause readmission, relapse, and mortality 30 days after completion of therapy.
Results
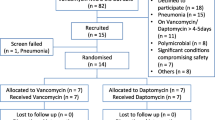
Sixty-three patients were included. Significantly fewer patients were able to achieve a therapeutic goal on IIV compared to CIV (52.4% vs. 82.5%, p < 0.01). Patients on IIV took 3.6 days, on average, to reach the target goal, compared to 1.9 days when patients were switched to CIV (95% confidence interval, 0.48–3.04, p < 0.01). Six patients experienced adverse events on IIV, and 15 patients experienced adverse events on CIV (IIV 9.5%, CIV 23.8%, p = 0.035). One patient experienced relapse of infection, and six patients (9.5%) were readmitted 30 days after completion of therapy. There were no deaths in the cohort.
Conclusion
For MRSA bacteremia, CIV enabled patients to achieve the AUC/MIC goal significantly faster than when patients received IIV. Furthermore, patients who were unable to achieve a therapeutic trough on IIV became therapeutic once switched to CIV.


Similar content being viewed by others
Availability of data and materials
This declaration is not applicable.
References
Rybak MJ, Lomaestro BM, Rotscahfer JC et al (2009) Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 49(3):325–327. https://doi.org/10.1086/600877
Rybak MJ, Le J, Lodise TP et al (2020) Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 77(11):835–864. https://doi.org/10.1093/ajhp/zxaa036
Hao J-J, Chen H, Zhou J-X (2016) Continuous versus intermittent infusion of vancomycin in adult patients: a systematic review and meta-analysis. Int J Antimicrob Agents 47(1):28–35. https://doi.org/10.1016/j.ijantimicag.2015.10.019
Wysocki M, Delatour F, Faurisson F et al (2001) Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 45(9):2460–2467. https://doi.org/10.1128/AAC.45.9.2460-2467.2001
Schlobohm CJ, Zhu E, Duby JJ (2021) Continuous Infusion versus Intermittent Infusion Vancomycin in a Burn Center Intensive Care Unit. Burns 47(7):1495–1501. https://doi.org/10.1016/j.burns.2021.08.016
Flannery AH, Bissell BD, Bastin MT, Morris PE, Neyra JA (2020) Continuous versus intermittent infusion of vancomycin and the risk of acute kidney injury in critically ill adults: a systematic review and meta-analysis. Crit Care Med 48(6):912–18. https://doi.org/10.1097/CCM.0000000000004326
Singbartl K, Kellum JA (2012) AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int 81(9):819–25. https://doi.org/10.1038/ki.2011.339
Neely MN, Kato L, Youn G, Kraler L, Bayard D, van Guilder M, Schumitzky A, Yamada W, Jones B, Minejima E (2018) Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. AntimicrobAgents Chemother 62(2):e02042–17. https://doi.org/10.1128/AAC.02042-17
Linder A, Fjell C, Levin A, Walley KR, Russell JA, Boyd JH (2014) Small acute increases in serum creatinine are associated with decreased long-term survival in the critically ill. Am J Respir Crit Care Med 189(9):1075–1081. https://doi.org/10.1164/rccm.201311-2097OC
Spapen HD, Janssen van Doorn K, Diltoer M, Verbrugghe W, Jacobs R, Dobbeleir N, Honoré PM, Jorens PG (2011) Retrospective evaluation of possible renal toxicity associated with continuous infusion of vancomycin in critically ill patients. Ann Intensive Care 1(1):26. https://doi.org/10.1186/2110-5820-1-26
Cianferoni S, Devigili A, Ocampos-Martinez E, Penaccini L, Scolletta S, Abdelhadii A, De Backer D et al (2013) Development of acute kidney injury during continuous infusion of vancomycin in septic patients. Infection 41(4):811–20. https://doi.org/10.1007/s15010-013-0460-9
Hutschala D, Kinstner C, Skhirdladze K, Thalhammer F, Müller M, Tschernko E (2009) Influence of vancomycin on renal function in critically ill patients after cardiac surgery: continuous versus intermittent infusion. Anesthesiology 111(2):356–65. https://doi.org/10.1097/ALN.0b013e3181a97272
Ray AS, Haikal A, Hammoud KA, Alan SL (2016) Vancomycin and the risk of AKI: a systematic review and meta-analysis. Clin J Am Soc Nephrol CJASN 11(12):2132–40. https://doi.org/10.2215/CJN.05920616
Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S (2013) Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ (Clinical Research Ed.) 346:e8525. https://doi.org/10.1136/bmj.e8525
van Hal SJ, Paterson DL, Lodise TP (2013) Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 57(2):734–44. https://doi.org/10.1128/AAC.01568-12
Jorgensen SC, Murray KP, Lagnf AM, Melvin S, Bhatia S, Shamim MD, Smith JR et al (2020) A multicenter evaluation of vancomycin-associated acute kidney injury in hospitalized patients with acute bacterial skin and skin structure infections. Infect Dis Ther 9(1):89–106. https://doi.org/10.1007/s40121-019-00278-1
Filippone EJ, Kraft WK, Farber JL (2017) The nephrotoxicity of vancomycin. Clin Pharmacol Ther 102(3):459–469. https://doi.org/10.1002/cpt.726
Miano TA, Hennessy S, Yang W et al (2022) Association of vancomycin plus piperacillin–tazobactam with early changes in creatinine versus cystatin C in critically ill adults: a prospective cohort study. Intensive Care Med 48(9):1144–1155. https://doi.org/10.1007/s00134-022-06811-0
Author information
Authors and Affiliations
Contributions
D.G., M.C., C.W., and D.A contributed to the design and implementation of the research. D.G., M.C., and C.W. wrote the main manuscript text and prepared all figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This protocol was approved by the Institutional Review Board (STUDY00005330), and the requirement for patient consent was waived.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gilliam, D., Acosta, D., Carvour, M.L. et al. Retrospective review of intermittent and continuous infusion vancomycin for methicillin-resistant Staphylococcus aureus bacteremia. Eur J Clin Pharmacol 80, 75–81 (2024). https://doi.org/10.1007/s00228-023-03585-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03585-2